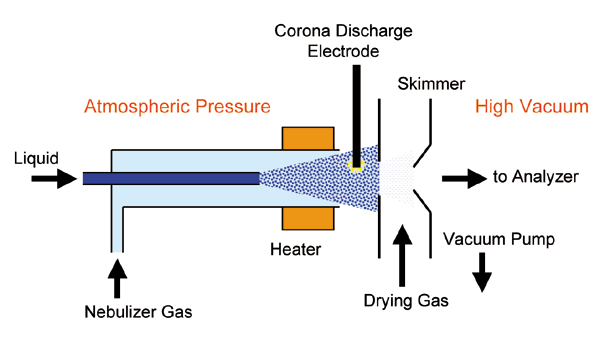
Definition of Mass Spectrometry
Mass Spectrometry is a very important analytical technique that is responsible for measuring the mass to charge ratio (m/Q) of the ions. The results are depicted in the form of a mass spectrum. It is defined as a plot of intensity having a mass to charge ratio as the function of it.
This technique is used in several fields. The samples used in mass spectrometry are usually pure but complex mixtures can also be used. The applications of mass spectrometry include the determination of isotopes, the mass of particles, and interpreting the chemical nature and structure of molecules.
A mass spectrometer is composed of an ionizing chamber, a mass analyzer and finally a detector. The sample used in this can be in the form of solid, liquid, and or gas. This is then ionized by the bombardment of electrons. These ions are then directed towards the analyzer with the help of electric and magnetic fields and separated based on their mass to charge ratio.
The ions having the same ratio will undergo the same amount of deflection. These charged particles are then detected by the aid of an electron multiplier (amplifier) and the results are displayed in the form of a mass spectrum.
View More Organic Chemistry Definitions
Related Questions of Mass Spectrometry
For your work in a mass spectrometry lab, you are investigating
Small organic molecules known as plasticizers are added to polymers to make
Compare the structures of cyclohexane and 2-methyl-2-
Consider the following sequence of reactions: / a
The following is the proposed structure of a blue fabric dye,
The following two isomers were each subjected to mass spectrometric analysis.
Pinolenic acid (C17H29CO2H) is an unbranched carboxylic acid with three
In what area does mass spectrometry currently have its greatest application for
Mass spectrometry is an extremely versatile detection system for gas chromatography.
Show All