Definition of Molecular Structure
The location of all the atoms present in a molecule of a chemical compound is being described by the molecular structure. In this kind of structure, the electron-pair geometries are not depicted, hence we are unable to tell whether there is a lone pair present around the central atom of the molecule or not.
Examples of Molecular structure:
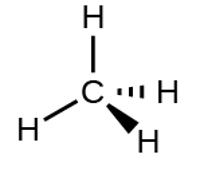
Consider the example of a methane molecule. The molecular formula of methane is CH4 and it is one of the major components of natural gas. The molecular structure of the methane depicts that it has a tetrahedral geometry. The carbon is the central atom and four hydrogen atoms are bonded to it in a tetrahedral manner.
View More General Chemistry Definitions
Related Questions of Molecular Structure
Using the VSEPR theory, predict the molecular structure of each of
Why is the molecular structure of H2O nonlinear, whereas that of
Using the VSEPR theory, predict the molecular structure of each of
Using the VSEPR theory, predict the molecular structure of each of
The formulas of several chemical substances are given in the table below
Using the VSEPR theory, predict the molecular structure of each of
For each of the following molecules, predict both the molecular structure
Consider two specimens of ideal gas at the same temperature. Specimen
What general principles determine the molecular structure (shape) of a
Using the VSEPR theory, predict the molecular structure of each of
Show All