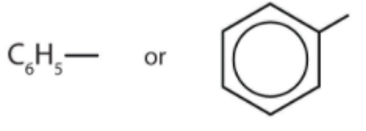
Definition of Phenyl Group
A phenyl group is formed when one atom of hydrogen is removed from the ring structure of the benzene. In this way, the molecular structure turns from C6H6 to C6H5. The hydrogen removed can be replaced by a number of elements and compounds. The phenyl group, therefore, belongs to the class of organic compounds which are aromatic in nature.
The phenyl group has a hexagonal planar structure and the length of the bonds between all the carbon atoms remains the same and it is 1.4 angstrom. The phenyl group is hydrophobic and is an electron-withdrawing group.
View More General Chemistry Definitions
Related Questions of General Chemistry
Which of the following statement(s) is(are)
Students approaching the study of chemistry must learn certain basic facts (
Suppose 325 mL of 0.150 M NaOH is needed for
When ethanol (grain alcohol, C2H5OH) is burned in oxygen
Read the “Chemistry in Focus” segment A Mystifying Problem,
Is the process represented below a physical or chemical change?
Why is the ability to solve problems important in the study of
Discuss several political, social, or personal considerations that might affect
Although reviewing your lecture notes and reading your textbook are important,
Evaluate each of the following and write the answer to the appropriate
Show All