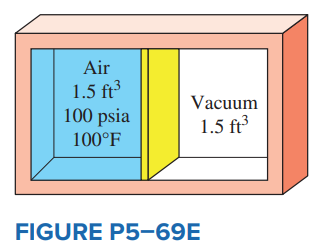
Question: A 3-ft3 adiabatic rigid container is
A 3-ft3 adiabatic rigid container is divided into two equal volumes by a thin membrane, as shown in Fig. P5–69E. Initially, one of these chambers is filled with air at 100 psia and 100°F while the other chamber is evacuated. Determine the internal energy change of the air when the membrane is ruptured. Also determine the final air pressure in the container.
> A 4-L pressure cooker has an operating pressure of 175 kPa. Initially, one-half of the volume is filled with liquid and the other half with vapor. If it is desired that the pressure cooker not run out of liquid water for 75 min, determine the highest rat
> A 3-ft3 rigid tank initially contains saturated water vapor at 300°F. The tank is connected by a valve to a supply line that carries steam at 200 psia and 400°F. Now the valve is opened, and steam is allowed to enter the tank. Heat transfer takes place w
> A 0.2-m3 rigid tank equipped with a pressure regulator contains steam at 2 MPa and 300°C. The steam in the tank is now heated. The regulator keeps the steam pressure constant by letting out some steam, but the temperature inside rises. Determine the amou
> A 2-m3 rigid tank initially contains air at 100 kPa and 22°C. The tank is connected to a supply line through a valve. Air is flowing in the supply line at 600 kPa and 22°C. The valve is opened, and air is allowed to enter the tank u
> Consider a 35-L evacuated rigid bottle that is surrounded by the atmosphere at 100 kPa and 22°C. A valve at the neck of the bottle is now opened and the atmospheric air is allowed to flow into the bottle. The air trapped in the bottle eventual
> A 2-m3 rigid insulated tank initially containing saturated water vapor at 1 MPa is connected through a valve to a supply line that carries steam at 400°C. Now the valve is opened, and steam is allowed to flow slowly into the tank until the pre
> Both a gage and a manometer are attached to a gas tank to measure its pressure. If the reading on the pressure gage is 80 kPa, determine the distance between the two fluid levels of the manometer if the fluid is (a) mercury (ρ = 13,600 kg/m3)
> A rigid, insulated tank that is initially evacuated is connected through a valve to a supply line that carries steam at 4 MPa. Now the valve is opened, and steam is allowed to flow into the tank until the pressure reaches 4 MPa, at which point the valve
> An insulated rigid tank is initially evacuated. A valve is opened, and atmospheric air at 95 kPa and 17°C enters the tank until the pressure in the tank reaches 95 kPa, at which point the valve is closed. Determine the final temperature of the air in the
> Air enters the duct of an air-conditioning system at 15 psia and 50°F at a volume flow rate of 450 ft3/min. The diameter of the duct is 10 in, and heat is transferred to the air in the duct from the surroundings at a rate of 2 Btu/s. Determine (a) the ve
> Reconsider Prob. 6–99. Using appropriate software, investigate the effect of the exit cross sectional area of the hair dryer on the exit velocity. Let the exit area vary from 25 to 75 cm2. Plot the exit velocity against the exit cross-s
> A 2-m3 rigid tank initially contains air whose density is 1.18 kg/m3. The tank is connected to a high-pressure supply line through a valve. The valve is opened, and air is allowed to enter the tank until the density in the tank rises to 5.30 kg/m3. Deter
> Name four physical quantities that are conserved and two quantities that are not conserved during a process.
> A mass of 12 kg of saturated refrigerant-134a vapor is contained in a piston–cylinder device at 240 kPa. Now 300 kJ of heat is transferred to the refrigerant at constant pressure while a 110-V source supplies current to a resistor withi
> A mass of 3 kg of saturated liquid–vapor mixture of water is contained in a piston–cylinder device at 160 kPa. Initially, 1 kg of the water is in the liquid phase and the rest is in the vapor phase. Heat is now transfe
> A well-insulated, rigid vessel contains 3 kg of saturated liquid water at 40°C. The vessel also contains an electrical resistor that draws 10 A when 50 V are applied. Determine the final temperature in the vessel after the resistor has been operating for
> Nitrogen at 100 kPa and 25°C in a rigid vessel is heated until its pressure is 300 kPa. Calculate the work done and the heat transferred during this process, in kJ/kg.
> The piston of a vertical piston–cylinder device containing a gas has a mass of 60 kg and a cross sectional area of 0.04 m2, as shown in Fig. P2–43. The local atmospheric pressure is 0.97 bar, and the gravitational acce
> Air is expanded in a polytropic process with n = 1.2 from 1 MPa and 400°C to 110 kPa in a piston–cylinder device. Determine the final temperature of the air.
> Air in the amount of 2 lbm is contained in a well-insulated, rigid vessel equipped with a stirring paddle wheel. The initial state of this air is 30 psia and 60°F. How much work, in Btu, must be transferred to the air with the paddle wheel to
> A mass of 0.2 kg of saturated refrigerant-134a is contained in a piston–cylinder device at 200 kPa. Initially, 75 percent of the mass is in the liquid phase. Now heat is transferred to the refrigerant at constant pressure until the cylinder contains vapo
> Consider a piston–cylinder device that contains 0.5 kg air. Now heat is transferred to the air at constant pressure and the air temperature increases by 5°C. Determine the expansion work done during this process.
> A rigid tank contains a gas mixture with a specific heat of cv = 0.748 kJ/kg·K. The mixture is cooled from 200 kPa and 200°C until its pressure is 100 kPa. Determine the heat transfer during this process, in kJ/kg.
> The temperature of air changes from 0 to 10°C while its velocity changes from zero to a final velocity, and its elevation changes from zero to a final elevation. At which values of final air velocity and final elevation will the internal, kinetic, and po
> A frictionless piston–cylinder device contains 16 lbm of superheated water vapor at 40 psia and 600°F. Steam is now cooled at constant pressure until 70 percent of it, by mass, condenses. Determine the work done during this process.
> Consider a classroom that is losing heat to the outdoors at a rate of 12,000 kJ/h. If there are 40 students in class, each dissipating sensible heat at a rate of 84 W, determine if it is necessary to turn the heater in the classroom on to prevent the roo
> Which of two gases—neon or air—produces more work when expanded from P1 to P2 in a closed system polytropic process with n = 1.2?
> Which of two gases—neon or air—requires less work when compressed in a closed system from P1 to P2 using a polytropic process with n = 1.5?
> Reconsider Prob. 2–41. Using appropriate software, investigate the effect of the spring force in the range of 0 to 500 N on the pressure inside the cylinder. Plot the pressure against the spring force, and discuss the results. Data fro
> If you ever slapped someone or got slapped yourself, you probably remember the burning sensation. Imagine you had the unfortunate occasion of being slapped by an angry person, which caused the temperature of the affected area of your face to rise by 2.4°
> Reconsider Prob. 5–84. Using appropriate software, investigate the effect of the mass of the heat sink on the maximum device temperature. Let the mass of the heat sink vary from 0 to 1 kg. Plot the maximum temperature against the mass of the heat sink, a
> An electronic device dissipating 25 W has a mass of 20 g and a specific heat of 850 J/kg·°C. The device is lightly used, and it is on for 5 min and then off for several hours, during which it cools to the ambient temperature of 25°C. Determine the highes
> Long cylindrical steel rods (ρ = 7833 kg/m3 and cp = 0.465 kJ/kg·°C) of 8 cm diameter are heat treated by drawing them at a velocity of 2 m/min through an oven maintained at 900°C. If the rods enter the oven at 30°C and leave at a mean temperature of 500
> In a production facility, 1.6-in-thick 2-ft × 2-ft square brass plates (ρ = 532.5 lbm/ft3 and cp = 0.091 Btu/lbm·°F) that are initially at a uniform temperature of 75°F are heated by passing the
> Stainless steel ball bearings (ρ = 8085 kg/m3 and cp = 0.480 kJ/kg·°C) having a diameter of 1.2 cm are to be quenched in water at a rate of 800 per minute. The balls leave the oven at a uniform temperature of 900°C and are exposed to air at 25°C for a wh
> Consider a 1000-W iron whose base plate is made of 0.5-cm-thick aluminum alloy 2024-T6 (ρ = 2770 kg/m3 and cp = 875 J/kg·°C). The base plate has a surface area of 0.03 m2. Initially, the iron is in thermal equilibrium
> A mass of 5 kg of saturated water vapor at 150 kPa is heated at constant pressure until the temperature reaches 200°C. Calculate the work done by the steam during this process.
> An ordinary egg can be approximated as a 5.5-cm-diameter sphere. The egg is initially at a uniform temperature of 8°C and is dropped into boiling water at 97°C. Taking the properties of the egg to be ρ = 1020 kg/m3 and cp = 3.32 kJ/kg·°C, determine how m
> During a picnic on a hot summer day, all the cold drinks disappear quickly, and the only available drinks are those at the ambient temperature of 85°F. In an effort to cool a 12-fluid-oz drink in a can, a person grabs the can and starts shaking it in the
> A gas is contained in a vertical, frictionless piston–cylinder device. The piston has a mass of 3.2 kg and a cross-sectional area of 35 cm2. A compressed spring above the piston exerts a force of 150 N on the piston. If the atmospheric
> The state of liquid water is changed from 50 psia and 50°F to 2000 psia and 100°F. Determine the change in the internal energy and enthalpy of water on the basis of the (a) compressed liquid tables, (b) incompressible substance approximation and property
> A 1-kg block of iron is heated from 25 to 75°C. What is the change in the iron’s total internal energy and enthalpy?
> A piston–cylinder device, whose piston is resting on a set of stops, initially contains 3 kg of air at 200 kPa and 27°C. The mass of the piston is such that a pressure of 400 kPa is required to move it. Heat is now transferred to the air until its volume
> A piston–cylinder device whose piston is resting on top of a set of stops initially contains 0.5 kg of helium gas at 100 kPa and 25°C. The mass of the piston is such that 500 kPa of pressure is required to raise it. How much heat must be transferred to t
> A spring-loaded piston–cylinder device contains 5 kg of helium as the system, as shown in Fig. P5–73. This system is heated from 100 kPa and 20°C to 800 kPa and 160°C. Determine the heat transferr
> A piston–cylinder device contains 4 kg of argon at 250 kPa and 35°C. During a quasi-equilibrium, isothermal expansion process, 15 kJ of boundary work is done by the system, and 3 kJ of paddle wheel work is done on the system. Determine the heat transfer
> Reconsider Prob. 5–70. Using appropriate software, plot the process described in the problem on a P-V diagram, and investigate the effect of the polytropic exponent n on the boundary work and heat transfer. Let the polytropic exponent vary from 1.0 to 1.
> A piston–cylinder device contains 2.2 kg of nitrogen initially at 100 kPa and 25°C. The nitrogen is now compressed slowly in a polytropic process during which PV1.3 = constant until the volume is reduced by one-half. Determine the work done and the heat
> A piston–cylinder device with a set of stops initially contains 0.6 kg of steam at 1.0 MPa and 400°C. The location of the stops corresponds to 40 percent of the initial volume. Now the steam is cooled. Determine the compressi
> Solve Prob. 2–39 using appropriate software. Print out the entire solution, including the numerical results with proper units. Data from Prob. 2-39: The basic barometer can be used to measure the height of a building. If the barometric readings at the t
> How does the science of heat transfer differ from the science of thermodynamics?
> A mass of 15 kg of air in a piston–cylinder device is heated from 25 to 95°C by passing current through a resistance heater inside the cylinder. The pressure inside the cylinder is held constant at 300 kPa during the process,
> Air is contained in a variable-load piston–cylinder device equipped with a paddle wheel. Initially, air is at 400 kPa and 17°C. The paddle wheel is now turned by an external electric motor until 75 kJ/kg of work has been tran
> An insulated piston–cylinder device contains 100 L of air at 400 kPa and 25°C. A paddle wheel within the cylinder is rotated until 15 kJ of work is done on the air while the pressure is held constant. Determine the final temperature of the air. Neglect t
> Argon is compressed in a polytropic process with n = 1.2 from 120 kPa and 10°C to 800 kPa in a piston–cylinder device. Determine the work produced and heat transferred during this compression process, in kJ/kg.
> An insulated piston–cylinder device initially contains 0.3 m3 of carbon dioxide at 200 kPa and 27°C. An electric switch is turned on, and a 110-V source supplies current to a resistance heater inside the cylinder for a period of 10 min. The pressure is h
> A 4-m × 5-m × 6-m room is to be heated by a baseboard resistance heater. It is desired that the resistance heater be able to raise the air temperature in the room from 5 to 25°C within 17 min. Assuming no heat losses from the room and an atmospheric pres
> An ideal gas contained in a piston–cylinder device under goes an isothermal compression process which begins with an initial pressure and volume of 100 kPa and 0.6 m3, respectively. During the process there is a heat transfer of 60 kJ from the ideal gas
> An insulated rigid tank is divided into two equal parts by a partition. Initially, one part contains 4 kg of an ideal gas at 800 kPa and 50°C, and the other part is evacuated. The partition is now removed, and the gas expands into the entire t
> A 4-m × 5-m × 7-m room is heated by the radiator of a steam-heating system. The steam radiator transfers heat at a rate of 10,000 kJ/h, and a 100-W fan is used to distribute the warm air in the room. The rate of heat loss from t
> The volume of 1 kg of helium in a piston–cylinder device is initially 5 m3. Now helium is compressed to 2 m3 while its pressure is maintained constant at 130 kPa. Determine the initial and final temperatures of helium as well as the work required to comp
> The basic barometer can be used to measure the height of a building. If the barometric readings at the top and at the bottom of a building are 675 and 695 mmHg, respectively, determine the height of the building. Take the densities of air and mercury to
> A 10-ft3 tank contains oxygen initially at 14.7 psia and 80°F. A paddle wheel within the tank is rotated until the pressure inside rises to 20 psia. During the process 20 Btu of heat is lost to the surroundings. Determine the paddle-wheel work done. Negl
> 1 kg of oxygen is heated from 20 to 120°C. Determine the amount of heat transfer required when this is done during a (a) constant-volume process and (b) isobaric process.
> A 3-m3 rigid tank contains hydrogen at 250 kPa and 550 K. The gas is now cooled until its temperature drops to 350 K. Determine (a) the final pressure in the tank and (b) the amount of heat transfer.
> A piston–cylinder device containing carbon dioxide gas undergoes an isobaric process from 15 psia and 80°F to 200°F. Determine the work and the heat transfer associated with this process, in Btu/lbm.
> Nitrogen in a rigid vessel is cooled by rejecting 100 kJ/kg of heat. Determine the internal energy change of the nitrogen, in kJ/kg.
> Is it possible to compress an ideal gas isothermally in an adiabatic piston–cylinder device? Explain.
> Determine the enthalpy change Δh of oxygen, in Btu/lbm, as it is heated from 800 to 1500 R, using (a) the empirical specific heat equation as a function of temperature, (b) the cp value at the average temperature, and (c) the cp value at room temperature
> Determine the internal energy change Δu of hydrogen, in kJ/kg, as it is heated from 200 to 800 K, using (a) the empirical specific heat equation as a function of temperature, (b) the cv value at the average temperature, and (c) the cv value at room tempe
> A mass of 10 g of nitrogen is contained in the spring loaded piston–cylinder device shown in Fig. P5–51. The spring constant is 1 kN/m, and the piston diameter is 10 cm. When the spring exerts no force against the pist
> What is the change in the enthalpy, in kJ/kg, of oxygen as its temperature changes from 150 to 250°C? Is there any difference if the temperature change were from 0 to 100°C? Does the pressure at the beginning and end of this process have any effect on th
> The barometer of a mountain hiker reads 750 mbars at the beginning of a hiking trip and 650 mbars at the end. Neglecting the effect of altitude on local gravitational acceleration, determine the vertical distance climbed. Assume an average air density of
> Nitrogen at an initial state of 300 K, 150 kPa, and 0.2 m3 is compressed slowly in an isothermal process to a final pressure of 800 kPa. Determine the work done during this process.
> Neon is compressed from 100 kPa and 20°C to 500 kPa in an isothermal compressor. Determine the change in the specific volume and specific enthalpy of neon caused by this compression.
> What is the change in the internal energy, in Btu/lbm, of air as its temperature changes from 100 to 200°F? Is there any difference if the temperature were to change from 0 to 100°F?
> Is the relation Δh = mcp,avgΔT restricted to constant pressure processes only, or can it be used for any kind of process of an ideal gas?
> Is the relation Δu = mcv,avg ΔT restricted to constant volume processes only, or can it be used for any kind of process of an ideal gas?
> A fixed mass of an ideal gas is heated from 50 to 80°C (a) at constant volume and (b) at constant pressure. For which case do you think the energy required will be greater? Why?
> A fixed mass of an ideal gas is heated from 50 to 80°C at a constant volume of (a) 1 m3 and (b) 3 m3. For which case do you think the energy required will be greater? Why?
> A fixed mass of an ideal gas is heated from 50 to 80°C at a constant pressure of (a) 1 atm and (b) 3 atm. For which case do you think the energy required will be greater? Why?
> Is the energy required to heat air from 295 to 305 K the same as the energy required to heat it from 345 to 355 K? Assume the pressure remains constant in both cases.
> Two tanks (Tank A and Tank B) are separated by a partition. Initially Tank A contains 2 kg of steam at 1 MPa and 300°C while Tank B contains 3 kg of saturated liquid–vapor mixture at 150°C with a vapor mass fractio
> Consider a 1.75-m-tall man standing vertically in water and completely submerged in a pool. Determine the difference between the pressures acting at the head and at the toes of the man, in kPa.
> Reconsider Prob. 5–39. Using appropriate software, investigate the effect of the initial pressure of water on the final temperature in the tank. Let the initial pressure vary from 100 to 600 kPa. Plot the final temperature against the i
> Calculate the total work, in kJ, for process 1–3 shown in Fig. P5–4 when the system consists of 2 kg of nitrogen.
> An insulated tank is divided into two parts by a partition. One part of the tank contains 2.5 kg of compressed liquid water at 60°C and 600 kPa while the other part is evacuated. The partition is now removed, and the water expands to fill the
> A piston–cylinder device initially contains 0.6 m3 of saturated water vapor at 250 kPa. At this state, the piston is resting on a set of stops, and the mass of the piston is such that a pressure of 300 kPa is required to move it. Heat is now slowly trans
> Steam at 75 kPa and 8 percent quality is contained in a spring-loaded piston–cylinder device, as shown in Fig. P5–37, with an initial volume of 2 m3. Steam is now heated until its volume is 5 m3 and its pressure is 225
> A 40-L electrical radiator containing heating oil is placed in a 50-m3 room. Both the room and the oil in the radiator are initially at 10°C. The radiator with a rating of 2.4 kW is now turned on. At the same time, heat is lost from the room a
> An insulated piston–cylinder device contains 5 L of saturated liquid water at a constant pressure of 175 kPa. Water is stirred by a paddle wheel while a current of 8 A flows for 45 min through a resistor placed in the water. If one-half
> 2 kg of saturated liquid water at 150°C is heated at constant pressure in a piston–cylinder device until it is saturated vapor. Determine the heat transfer required for this process.
> A piston–cylinder device contains 0.5 lbm of water initially at 120 psia and 2 ft3. Now 200 Btu of heat is transferred to the water while its pressure is held constant. Determine the final temperature of the water. Also, show the process on a T-v diagram
> A piston–cylinder device contains 5 kg of refrigerant 134a at 800 kPa and 70°C. The refrigerant is now cooled at constant pressure until it exists as a liquid at 15°C. Determine the amount of heat loss and show the process on a T-v diagram with respect t
> The absolute pressure in water at a depth of 9 m is read to be 185 kPa. Determine (a) the local atmospheric pressure and (b) the absolute pressure at a depth of 5 m in a liquid whose specific gravity is 0.85 at the same location.
> A rigid 1-ft3 vessel contains R-134a originally at –20°F and 27.7 percent quality. The refrigerant is then heated until its temperature is 100°F. Calculate the heat transfer required to do this.
> A rigid 10-L vessel initially contains a mixture of liquid water and vapor at 100°C with 12.3 percent quality. The mixture is then heated until its temperature is 180°C. Calculate the heat transfer required for this process.
> An ideal gas at a given state expands to a fixed final volume first at constant pressure and then at constant temperature. For which case is the work done greater?
> A 20-ft3 rigid tank initially contains saturated refrigerant 134a vapor at 160 psia. As a result of heat transfer from the refrigerant, the pressure drops to 50 psia. Show the process on a P-v diagram with respect to saturation lines, and determine (a) t
> A 0.5-m3 rigid tank contains refrigerant-134a initially at 160 kPa and 40 percent quality. Heat is now transferred to the refrigerant until the pressure reaches 700 kPa. Determine (a) the mass of the refrigerant in the tank and (b) the amount of heat tra

