Question: A clean nickel surface is exposed to
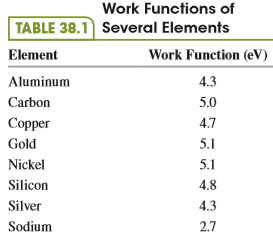
A clean nickel surface is exposed to light of wavelength 235 nm. What is the maximum speed of the photoelectrons emitted from this surface? Use Table 38.1.
From table 38.1:
Transcribed Image Text:
Work Functions of TABLE 38.1 Several Elements Element Work Function (eV) Aluminum 4.3 Carbon 5.0 Сорper 4.7 Gold 5.1 Nickel 5.1 Silicon 4.8 Silver 4.3 Sodium 2.7
> Suppose that the uncertainty of position of an electron is equal to the radius of the n = 1 Bohr orbit for hydrogen. Calculate the simultaneous minimum uncertainty of the corresponding momentum component, and compare this with the magnitude of the moment
> High-speed electrons are used to probe the interior structure of the atomic nucleus. For such electrons the expression l = h/p still holds, but we must use the relativistic expression for momentum, p = mv/ 1 −v2/c2 . a. Show that the
> What is the de Broglie wavelength of a red blood cell, with mass 1.00 * 10-11 g, that is moving with a speed of 0.400 cm/s? Do we need to be concerned with the wave nature of the blood cells when we describe the flow of blood in the body?
> A beam of 40-eV electrons traveling in the +xdirection passes through a slit that is parallel to the y-axis and 5.0 µm wide. The diffraction pattern is recorded on a screen 2.5 m from the slit. a. What is the de Broglie wavelength of the el
> a. Write the Planck distribution law in terms of the frequency f, rather than the wavelength λ, to obtain I(f). b. Show that where I(λ) is the Planck distribution formula of Eq. (39.24). Hint: Change the integration variable
> In the Bohr model of the hydrogen atom, what is the de Broglie wavelength of the electron when it is in a. the n = 1 level and b. the n = 4 level? In both cases, compare the de Broglie wavelength to the circumference 2prn of the orbit.
> a. What is the smallest amount of energy in electron volts that must be given to a hydrogen atom initially in its ground level so that it can emit the Ha line in the Balmer series? b. How many different possibilities of spectral-line emissions are there
> Repeat Exercise 34.38 for the case in which the lens is diverging, with a focal length of -48.0 cm. From Exercise 34.38: A converging lens with a focal length of 12.0 cm forms a virtual image 8.00 mm tall, 17.0 cm to the right of the lens. Determine the
> A large number of hydrogen atoms are in thermal equilibrium. Let n2/n1 be the ratio of the number of atoms in an n = 2 excited state to the number of atoms in an n = 1 ground state. At what temperature is n2 /n1 equal to a. 10-12; b. 10-8; c. 10-4? d
> Calculate the de Broglie wavelength of a 5.00-g bullet that is moving at 340 m/s. Will the bullet exhibit wavelike properties?
> What is the de Broglie wavelength for an electron with speed a. v = 0.480c and b. v = 0.960c? (Hint: Use the correct relativistic expression for linear momentum if necessary.)
> a. A nonrelativistic free particle with mass m has kinetic energy K. Derive an expression for the de Broglie wavelength of the particle in terms of m and K. b. What is the de Broglie wavelength of an 800-eV electron?
> An electron is moving with a speed of 8.00 * 106 m/s. What is the speed of a proton that has the same de Broglie wavelength as this electron?
> a. The uncertainty in the y-component of a proton’s position is 2.0 * 10-12 m. What is the minimum uncertainty in a simultaneous measurement of the y-component of the proton’s velocity? b. The uncertainty in the z-component of an electron’s velocity is
> The wavelength 10.0 mm is in the infrared region of the electromagnetic spectrum, whereas 600 nm is in the visible region and 100 nm is in the ultraviolet. What is the temperature of an ideal blackbody for which the peak wavelength lm is equal to each of
> The shortest visible wavelength is about 400 nm. What is the temperature of an ideal radiator whose spectral emittance peaks at this wavelength?
> An alpha particle (m = 6.64 * 10-27 kg) emitted in the radioactive decay of uranium-238 has an energy of 4.20 MeV. What is its de Broglie wavelength?
> An electron has a de Broglie wavelength of 2.80 * 10-10 m. Determine a. the magnitude of its momentum and b. its kinetic energy (in joules and in electron volts).
> Why do you think the development of Newtonian mechanics preceded the more refined relativistic mechanics by so many years?
> Find the longest and shortest wavelengths in the Lyman and Paschen series for hydrogen. In what region of the electromagnetic spectrum does each series lie?
> a. Using the Bohr model, calculate the speed of the electron in a hydrogen atom in the n = 1, 2, and 3 levels. b. Calculate the orbital period in each of these levels. c. The average lifetime of the first excited level of a hydrogen atom is 1.0 * 10-8
> A hydrogen atom initially in its ground level absorbs a photon, which excites the atom to the n = 3 level. Determine the wavelength and frequency of the photon.
> For crystal diffraction experiments (discussed in Section 39.1), wavelengths on the order of 0.20 nm are often appropriate. Find the energy in electron volts for a particle with this wavelength if the particle is a. a photon; b. an electron; c. an alp
> A hydrogen atom is in a state with energy -1.51 eV. In the Bohr model, what is the angular momentum of the electron in the atom, with respect to an axis at the nucleus?
> The silicon–silicon single bond that forms the basis of the mythical silicon-based creature the Horta has a bond strength of 3.80 eV. What wavelength of photon would you need in a (mythical) phasor disintegration gun to destroy the Horta?
> A CD-ROM is used instead of a crystal in an electron diffraction experiment. The surface of the CD-ROM has tracks of tiny pits with a uniform spacing of 1.60 mm. a. If the speed of the electrons is 1.26 * 104 m/s, at which values of θ will the m = 1 and
> a. In an electron microscope, what accelerating voltage is needed to produce electrons with wavelength 0.0600 nm? b. If protons are used instead of electrons, what accelerating voltage is needed to produce protons with wavelength 0.0600 nm? (Hint: In e
> A beam of neutrons that all have the same energy scatters from atoms that have a spacing of 0.0910 nm in the surface plane of a crystal. The m = 1 intensity maximum occurs when the angle θ in Fig. 39.2 is 28.6°. What is the kinetic energy (in electron vo
> Through what potential difference must electrons be accelerated if they are to have a. the same wavelength as an x ray of wavelength 0.220 nm and b. the same energy as the x ray in part (a)?
> In principle, does a hot gas have more mass than the same gas when it is cold? Explain. In practice, would this be a measurable effect? Explain.
> a. An electron moves with a speed of 4.70 * 106 m/s. What is its de Broglie wavelength? b. A proton moves with the same speed. Determine its de Broglie wavelength.
> Elements in the gaseous state emit line spectra with well-defined wavelengths. But hot solid bodies always emit a continuous spectrum—that is, a continuous smear of wavelengths. Can you account for this difference?
> How might the energy levels of an atom be measured directly—that is, without recourse to analysis of spectra?
> Galaxies tend to be strong emitters of Lyman-α photons (from the n = 2 to n = 1 transition in atomic hydrogen). But the intergalactic medium—the very thin gas between the galaxies— tends to absorb Lyman-α photons. What can you infer from these observatio
> Does a photon have a de Broglie wavelength? If so, how is it related to the wavelength of the associated electromagnetic wave? Explain.
> If a proton and an electron have the same kinetic energy, which has the longer de Broglie wavelength? Explain.
> Equation (39.30) states that the energy of a system can have uncertainty. Does this mean that the principle of conservation of energy is no longer valid? Explain.
> Does the uncertainty principle have anything to do with marksmanship? That is, is the accuracy with which a bullet can be aimed at a target limited by the uncertainty principle? Explain.
> Why go through the expense of building an electron microscope for studying very small objects such as organic molecules? Why not just use extremely short electromagnetic waves, which are much cheaper to generate?
> You have been asked to design a magnet system to steer a beam of 54-eV electrons like those described in Example 39.1 (Section 39.1). The goal is to be able to direct the electron beam to a specific target location with an accuracy of ±1.0
> A high-speed train passes a train platform. Larry is a passenger on the train, Adam is standing on the train platform, and David is riding a bicycle toward the platform in the same direction as the train is traveling. Compare the length of a train car as
> Do the planets of the solar system obey a distance law (rn = n2r1) as the electrons of the Bohr atom do? Should they? Why (or why not)? (Consult Appendix F for the appropriate distances.)
> If a proton and an electron have the same speed, which has the longer de Broglie wavelength? Explain.
> a. Show that in the Bohr model, the frequency of revolution of an electron in its circular orbit around a stationary hydrogen nucleus is f = me4/4ϵ02n3h3. b. In classical physics, the frequency of revolution of the electron is equal to the f
> You have entered a contest in which the contestants drop a marble with mass 20.0 g from the roof of a building onto a small target 25.0 m below. From uncertainty considerations, what is the typical distance by which you will miss the target, given that y
> A pulsed dye laser emits light of wavelength 585 nm in 450-ms pulses. Because this wavelength is strongly absorbed by the hemoglobin in the blood, the method is especially effective for removing various types of blemishes due to blood, such as port-wine–
> While interacting with molecules (mainly water) in the tumor tissue, each Compton electron or photoelectron causes a series of ionizations, each of which takes about 40 eV. Estimate the maximum number of ionizations that one photon generated by this line
> When ultraviolet light with a wavelength of 400.0 nm falls on a certain metal surface, the maximum kinetic energy of the emitted photoelectrons is measured to be 1.10 eV. What is the maximum kinetic energy of the photoelectrons when light of wavelength 3
> What would the minimum work function for a metal have to be for visible light (380–750 nm) to eject photoelectrons?
> A laser produces light of wavelength 625 nm in an ultrashort pulse. What is the minimum duration of the pulse if the minimum uncertainty in the energy of the photons is 1.0%?
> The cornea behaves as a thin lens of focal length approximately 1.8 cm, although this varies a bit. The material of which it is made has an index of refraction of 1.38, and its front surface is convex, with a radius of curvature of 5.0 mm. a. If this fo
> A horizontal beam of laser light of wavelength 585 nm passes through a narrow slit that has width 0.0620 mm. The intensity of the light is measured on a vertical screen that is 2.00 m from the slit. a. What is the minimum uncertainty in the vertical com
> An ultrashort pulse has a duration of 9.00 fs and produces light at a wavelength of 556 nm. What are the momentum and momentum uncertainty of a single photon in the pulse?
> A 16.0-cm-long pencil is placed at a 45.0° angle, with its center 15.0 cm above the optic axis and 45.0 cm from a lens with a 20.0-cm focal length as shown in Fig. P34.106. (Note that the figure is not drawn to scale.) Assume that the diameter
> X rays with an initial wavelength of 0.900 * 10-10 m undergo Compton scattering. For what scattering angle is the wavelength of the scattered x rays greater by 1.0% than that of the incident x rays?
> A photon scatters in the backward direction (ɸ = 180°) from a free proton that is initially at rest. What must the wavelength of the incident photon be if it is to undergo a 10.0% change in wavelength as a result of the scattering?
> The human eye is most sensitive to green light of wavelength 505 nm. Experiments have found that when people are kept in a dark room until their eyes adapt to the darkness, a single photon of green light will trigger receptor cells in the rods of the ret
> If a photon of wavelength 0.04250 nm strikes a free electron and is scattered at an angle of 35.0° from its original direction, find a. the change in the wavelength of this photon; b. the wavelength of the scattered light; c. the change in energy of th
> A photon with wavelength λ = 0.1385 nm scatters from an electron that is initially at rest. What must be the angle between the direction of propagation of the incident and scattered photons if the speed of the electron immediately after the collision is
> X rays are produced in a tube operating at 24.0 kV. After emerging from the tube, x rays with the minimum wavelength produced strike a target and undergo Compton scattering through an angle of 45.0°. a. What is the original x-ray wavelength? b. What is
> An x ray with a wavelength of 0.100 nm collides with an electron that is initially at rest. The x ray’s final wavelength is 0.110 nm. What is the final kinetic energy of the electron?
> The average life span in the United States is about 70 years. Does this mean that it is impossible for an average person to travel a distance greater than 70 light-years away from the earth? (A light-year is the distance light travels in a year.) Explain
> A certain very nearsighted person cannot focus on anything farther than 36.0 cm from the eye. Consider the simplified model of the eye described in Exercise 34.50. If the radius of curvature of the cornea is 0.75 cm when the eye is focusing on an object
> In one form of cataract surgery the person’s natural lens, which has become cloudy, is replaced by an artificial lens. The refracting properties of the replacement lens can be chosen so that the person’s eye focuses on distant objects. But there is no ac
> A camera with a 90-mm-focal-length lens is focused on an object 1.30 m from the lens. To refocus on an object 6.50 m from the lens, by how much must the distance between the lens and the sensor be changed? To refocus on the more distant object, is the le
> a. what is the minimum potential difference between the filament and the target of an x-ray tube if the tube is to produce x rays with a wavelength of 0.150 nm? b. What is the shortest wavelength produced in an x-ray tube operated at 30.0 kV?
> The smallest object we can resolve with our eye is limited by the size of the light receptor cells in the retina. In order for us to distinguish any detail in an object, its image cannot be any smaller than a single retinal cell. Although the size depend
> Protons are accelerated from rest by a potential difference of 4.00 kV and strike a metal target. If a proton produces one photon on impact, what is the minimum wavelength of the resulting x rays? How does your answer compare to the minimum wavelength if
> The cathode-ray tubes that generated the picture in early color televisions were sources of x rays. If the acceleration voltage in a television tube is 15.0 kV, what are the shortest-wavelength x rays produced by the television?
> The photoelectric work function of potassium is 2.3 eV. If light that has a wavelength of 190 nm falls on potassium, find a. the stopping potential in volts; b. the kinetic energy, in electron volts, of the most energetic electrons ejected; c. the spe
> In a photoelectric-effect experiment, the photocurrent i for large positive values of VAC has the same value no matter what the light frequency f (provided that f is higher than the threshold frequency f0). Explain why.
> Human skin is relatively insensitive to visible light, but ultraviolet radiation can cause severe burns. Does this have anything to do with photon energies? Explain.
> A double-convex thin lens has surfaces with equal radii of curvature of magnitude 2.50 cm. Using this lens, you observe that it forms an image of a very distant tree at a distance of 1.87 cm from the lens. What is the index of refraction of the lens?
> Would you expect effects due to the photon nature of light to be generally more important at the low-frequency end of the electromagnetic spectrum (radio waves) or at the high-frequency end (x rays and gamma rays)? Why?
> According to the photon model, light carries its energy in packets called quanta or photons. Why then don’t we see a series of flashes when we look at things?
> There is a certain probability that a single electron may simultaneously absorb two identical photons from a high-intensity laser. How would such an occurrence affect the threshold frequency and the equations of Section 38.1? Explain.
> Some lasers emit light in pulses that are only 10-12 s in duration. The length of such a pulse is (3 * 108 m/s)(10-12 s) = 3 * 10-4 m = 0.3 mm. Can pulsed laser light be as monochromatic as light from a laser that emits a steady, continuous beam? Explain
> Why must engineers and scientists shield against x-ray production in high-voltage equipment?
> The cornea of the eye has a radius of curvature of approximately 0.50 cm, and the aqueous humor behind it has an index of refraction of 1.35. The thickness of the cornea itself is small enough that we shall neglect it. The depth of a typical human eye is
> Can Compton scattering occur with protons as well as electrons? For example, suppose a beam of x rays is directed at a target of liquid hydrogen. (Recall that the nucleus of hydrogen consists of a single proton.) Compared to Compton scattering with elect
> Figure P34.81 shows an object and its image formed by a thin lens. a. What is the focal length of the lens, and what type of lens (converging or diverging) is it? b. What is the height of the image? Is it real or virtual? Figure P34.81 Image Objec
> In a photoelectric-effect experiment, which of the following will increase the maximum kinetic energy of the photoelectrons? a. Use light of greater intensity; b. use light of higher frequency; c. use light of longer wavelength; d. use a metal surfac
> In a particle accelerator a proton moves with constant speed 0.750c in a circle of radius 628 m. What is the net force on the proton?
> A photographic slide is to the left of a lens. The lens projects an image of the slide onto a wall 6.00 m to the right of the slide. The image is 80.0 times the size of the slide. a. How far is the slide from the lens? b. Is the image erect or inverted
> One of the wavelengths of light emitted by hydrogen atoms under normal laboratory conditions is l = 656.3 nm, in the red portion of the electromagnetic spectrum. In the light emitted from a distant galaxy this same spectral line is observed to be Doppler
> a. You want to use a lens with a focal length of 35.0 cm to produce a real image of an object, with the height of the image twice the height of the object. What kind of lens do you need, and where should the object be placed? b. Suppose you want a virtu
> In certain radioactive beta decay processes, the beta particle (an electron) leaves the atomic nucleus with a speed of 99.95% the speed of light relative to the decaying nucleus. If this nucleus is moving at 75.00% the speed of light in the laboratory re
> What should be the index of refraction of a transparent sphere in order for paraxial rays from an infinitely distant object to be brought to a focus at the vertex of the surface opposite the point of incidence?
> A nuclear bomb containing 12.0 kg of plutonium explodes. The sum of the rest masses of the products of the explosion is less than the original rest mass by one part in 104. a. How much energy is released in the explosion? b. If the explosion takes pla
> In our universe, the rest energy of an electron is approximately 8.2 * 10-14 J. What would it be in the alternate universe? a. 8.2 * 10-8 J; b. 8.2 * 10-26 J; c. 8.2 * 10-2 J; d. 0.82 J.
> Scientists working with a particle accelerator determine that an unknown particle has a speed of 1.35 * 108 m/s and a momentum of 2.52 * 10-19 kg.m/s. From the curvature of the particle’s path in a magnetic field, they also deduce that it has a positive
> The Russian physicist P. A. Cerenkov discovered that a charged particle traveling in a solid with a speed exceeding the speed of light in that material radiates electromagnetic radiation. (This is analogous to the sonic boom produced by an aircraft movin
> Physicists and engineers from around the world came together to build the largest accelerator in the world, the Large Hadron Collider (LHC) at the CERN Laboratory in Geneva, Switzerland. The machine accelerates protons to high kinetic energies in an unde
> Inside a spaceship flying past the earth at three-fourths the speed of light, a pendulum is swinging. a. If each swing takes 1.80 s as measured by an astronaut performing an experiment inside the spaceship, how long will the swing take as measured by a
> A spaceship is traveling toward the earth from the space colony on Asteroid 1040A. The ship is at the halfway point of the trip, passing Mars at a speed of 0.9c relative to the Mars frame of reference. At the same instant, a passenger on the spaceship re
> A light bulb is 3.00 m from a wall. You are to use a concave mirror to project an image of the bulb on the wall, with the image 3.50 times the size of the object. How far should the mirror be from the wall? What should its radius of curvature be?
> A concave mirror is to form an image of the filament of a headlight lamp on a screen 8.00 m from the mirror. The filament is 6.00 mm tall, and the image is to be 24.0 cm tall. a. How far in front of the vertex of the mirror should the filament be placed
> Where must you place an object in front of a concave mirror with radius R so that the image is erect and 2 1 2 times the size of the object? Where is the image?
> A spacecraft of the Trade Federation flies past the planet Coruscant at a speed of 0.600c. A scientist on Coruscant measures the length of the moving spacecraft to be 74.0 m. The spacecraft later lands on Coruscant, and the same scientist measures the le
> a. Through what potential difference does an electron have to be accelerated, starting from rest, to achieve a speed of 0.980c? b. What is the kinetic energy of the electron at this speed? Express your answer in joules and in electron volts.

