Question: A large tank of water has a
A large tank of water has a hose connected to it (Fig. P18.59). The tank is sealed at the top and has compressed air between the water surface and the top. When the water height h has the value 3.50 m, the absolute pressure p of the compressed air is 4.20 × 105 Pa. Assume that the air above the water expands at constant temperature, and take the atmospheric pressure to be 1.00 × 105 Pa.
Figure P18.59:
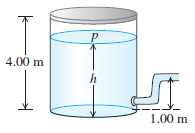
(a). What is the speed with which water flows out of the hose when h = 3.50 m?
(b). As water flows out of the tank, h decreases. Calculate the speed of flow for h = 3.00 m and for h = 2.00 m.
(c). At what value of h does the flow stop?
Transcribed Image Text:
4,00 m h 1.00 m
> A small metal band is slipped onto one of the tines of a tuning fork. As this band is moved closer and closer to the end of the tine, what effect does this have on the wavelength and frequency of the sound the tine produces? Why?
> A small fraction of the energy in a sound wave is absorbed by the air through which the sound passes. How does this modify the inverse-square relationship between intensity and distance from the source? Explain.
> The units of specific heat c are J/kg ∙ K, but the units of heat of fusion Lf or heat of vaporization Lv are simply J/kg. Why do the units of Lf and Lv not include a factor of (K)-1 to account for a temperature change?
> If the pressure amplitude of a sound wave is halved, by what factor does the intensity of the wave decrease? By what factor must the pressure amplitude of a sound wave be increased in order to increase the intensity by a factor of 16? Explain.
> Which has a more direct influence on the loudness of a sound wave: the displacement amplitude or the pressure amplitude? Explain.
> Two bodies made of the same material have the same external dimensions and appearance, but one is solid and the other is hollow. When their temperature is increased, is the overall volume expansion the same or different? Why?
> A glass flask whose volume is 1000.00 cm3 at 0.0 °C is completely filled with mercury at this temperature. When flask and mercury are warmed to 55.0 °C, 8.95 cm3 of mercury overflow. If the coefficient of volume expansion of mercury is 18.0 × 10-5 K-1, c
> Lane dividers on highways sometimes have regularly spaced ridges or ripples. When the tires of a moving car roll along such a divider, a musical note is produced. Why? Explain how this phenomenon could be used to measure the car’s speed.
> In a popular and amusing science demonstration, a person inhales helium and then his voice becomes high and squeaky. Why does this happen? (Warning: Inhaling too much helium can cause unconsciousness or death.)
> Symphonic musicians always “warm up” their wind instruments by blowing into them before a performance. What purpose does this serve?
> If you heat the air inside a rigid, sealed container until its Kelvin temperature doubles, the air pressure in the container will also double. Is the same thing true if you double the Celsius temperature of the air in the container? Explain.
> Would you expect the pitch (or frequency) of an organ pipe to increase or decrease with increasing temperature? Explain.
> The hero of a western movie listens for an oncoming train by putting his ear to the track. Why does this method give an earlier warning of the approach of a train than just listening in the usual way?
> Section 18.1 states that ordinarily, pressure, volume, and temperature cannot change individually without one affecting the others. Yet when a liquid evaporates, its volume changes, even though its pressure and temperature are constant. Is this inconsist
> The statistical quantities “average value” and “root-mean-square value” can be applied to any distribution. Figure P18.82 shows the scores of a class of 150 students on a 100-point q
> The vapor pressure of water (see Exercise 18.44) decreases as the temperature decreases. The table lists the vapor pressure of water at various temperatures: Exercise 18.44: The vapor pressure is the pressure of the vapor phase of a substance when it i
> A steel cylinder with rigid walls is evacuated to a high degree of vacuum; you then put a small amount of helium into the cylinder. The cylinder has a pressure gauge that measures the pressure of the gas inside the cylinder. You place the cylinder in var
> You blow across the open mouth of an empty test tube and produce the fundamental standing wave of the air column inside the test tube. The speed of sound in air is 344 m/s and the test tube acts as a stopped pipe. (a). If the length of the air column in
> A vertical cylinder of radius r contains an ideal gas and is fitted with a piston of mass m that is free to move (Fig. P18.79). The piston and the walls of the cylinder are frictionless, and the entire cylinder is placed in a constant-temperature bath. T
> Calculate the integral in Eq. (18.30), 0 ∞
> (a) Explain why in a gas of N molecules, the number of molecules having speeds in the finite interval v to v +
> (a). Calculate the total rotational kinetic energy of the molecules in 1.00 mol of a diatomic gas at 300 K. (b). Calculate the moment of inertia of an oxygen molecule (O2) for rotation about either the y- or z-axis shown in Fig. 18.18b. Treat the molecu
> (a). The temperature near the top of Jupiter’s multicolored cloud layer is about 140 K. The temperature at the top of the earth’s troposphere, at an altitude of about 20 km, is about 220 K. Calculate the rms speed of hydrogen molecules in both these envi
> (a). Show that a projectile with mass m can “escape” from the surface of a planet if it is launched vertically upward with a kinetic energy greater than mgRp, where g is the acceleration due to gravity at the planet’s surface and Rp is the planet’s radiu
> The surface of the sun has a temperature of about 5800 K and consists largely of hydrogen atoms. (a). Find the rms speed of a hydrogen atom at this temperature. (The mass of a single hydrogen atom is 1.67 × 10-27 kg.) (b). The escape speed
> It is possible to make crystalline solids that are only one layer of atoms thick. Such “two-dimensional” crystals can be created by depositing atoms on a very flat surface. (a). If the atoms in such a two-dimensional crystal can move only within the pla
> (a). What is the total random translational kinetic energy of 5.00 L of hydrogen gas (molar mass 2.016 g/mol) with pressure 1.01 × 105 Pa and temperature 300 K? (Hint: Use the procedure of Problem 18.67 as a guide.) Problem 18.67: You blow up a spheri
> A commonly used potential-energy function for the interaction of two molecules (see Fig. 18.8) is the Lennard-Jones 6-12 potential: where r is the distance between the centers of the molecules and U0 and R0 are positive constants. The corresponding for
> A pipe closed at both ends can have standing waves inside of it, but you normally don’t hear them because little of the sound can get out. But you can hear them if you are inside the pipe, such as someone singing in the shower. (a). Show that the wavele
> Consider a sound wave in air that has displacement amplitude 0.0200 mm. Calculate the pressure amplitude for frequencies of (a). 150 Hz; (b). 1500 Hz; (c). 15,000 Hz. In each case compare the result to the pain threshold, which is 30 Pa.
> (a). Compute the increase in gravitational potential energy for a nitrogen molecule (molar mass 28.0 g/mol) for an increase in elevation of 400 m near the earth’s surface. (b). At what temperature is this equal to the average kinetic energy of a nitroge
> You blow up a spherical balloon to a diameter of 50.0 cm until the absolute pressure inside is 1.25 atm and the temperature is 22.0 °C. Assume that all the gas is N2, of molar mass 28.0 g/mol. (a). Find the mass of a single N2 molecule. (b). How much tr
> Helium gas is in a cylinder that has rigid walls. If the pressure of the gas is 2.00 atm, then the root-mean-square speed of the helium atoms is vrms = 176 m/s. By how much (in atmospheres) must the pressure be increased to increase the vrms of the He at
> A sealed box contains a monatomic ideal gas. The number of gas atoms per unit volume is 5.00 × 1020 atoms/cm3, and the average translational kinetic energy of each atom is 1.80 × 10-23 J. (a). What is the gas pressure? (b). If the gas is neon (molar ma
> You have two identical containers, one containing gas A and the other gas B. The masses of these molecules are mA = 3.34 × 10-27 kg and mB = 5.34 × 10-26 kg. Both gases are under the same pressure and are at 10.0° C. (a). Which molecules (A or B) have g
> A person at rest inhales 0.50 L of air with each breath at a pressure of 1.00 atm and a temperature of 20.0 °C. The inhaled air is 21.0% oxygen. (a). How many oxygen molecules does this person inhale with each breath? (b). Suppose this person is now re
> Estimate the number of atoms in the body of a 50-kg physics student. Note that the human body is mostly water, which has molar mass 18.0 g/mol, and that each water molecule contains three atoms.
> During your mechanical engineering internship, you are given two uniform metal bars A and B, which are made from different metals, to determine their thermal conductivities. Measuring the bars, you determine that both have length 40.0 cm and uniform cros
> At a chemical plant where you are an engineer, a tank contains an unknown liquid. You must determine the liquid’s specific heat capacity. You put 0.500 kg of the liquid into an insulated metal cup of mass 0.200 kg. Initially the liquid and cup are at 20.
> As a physicist, you put heat into a 500.0-g solid sample at the rate of 10.0 kJ/min while recording its temperature as a function of time. You plot your data as shown in Fig. P17.111. (a). What is the latent heat of fusion for this solid? (b). What are
> The longest pipe found in most medium-size pipe organs is 4.88 m (16 ft) long. What is the frequency of the note corresponding to the fundamental mode if the pipe is (a). open at both ends, (b). open at one end and closed at the other?
> The icecaps of Greenland and Antarctica contain about 1.75% of the total water (by mass) on the earth’s surface; the oceans contain about 97.5%, and the other 0.75% is mainly groundwater. Suppose the icecaps, currently at an average temperature of about
> You have probably seen people jogging in extremely hot weather. There are good reasons not to do this! When jogging strenuously, an average runner of mass 68 kg and surface area 1.85 m2 produces energy at a rate of up to 1300 W, 80% of which is converted
> A metal sphere with radius 3.20 cm is suspended in a large metal box with interior walls that are maintained at 30.0° C. A small electric heater is embedded in the sphere. Heat energy must be supplied to the sphere at the rate of 0.660 J/s to maintain th
> A physicist uses a cylindrical metal can 0.250 m high and 0.090 m in diameter to store liquid helium at 4.22 K; at that temperature the heat of vaporization of helium is 2.09 × 104 J/kg. Completely surrounding the metal can are walls maintained at the te
> A light, plastic sphere with mass m = 9.00 g and density r = 4.00 kg/m3 is suspended in air by thread of negligible mass. (a). What is the tension T in the thread if the air is at 5.00oC and p = 1.00 atm? The molar mass of air is 28.8 g/mol. (b). How mu
> (a) When the air temperature is below 0° C, the water at the surface of a lake freezes to form an ice sheet. Why doesn’t freezing occur throughout the entire volume of the lake? (b). Show that the thickness of the ice sheet formed on the surface of a la
> The basal metabolic rate is the rate at which energy is produced in the body when a person is at rest. A 75-kg (165-lb) person of height 1.83 m (6 ft) has a body surface area of approximately 2.0 m2. (a). What is the net amount of heat this person could
> A brass rod 12.0 cm long, a copper rod 18.0 cm long, and an aluminum rod 24.0 cm long—each with cross-sectional area 2.30 cm3—are welded together end to end to form a rod 54.0 cm long, with copper as the middle section. The free end of the brass section
> Compute the ratio of the rate of heat loss through a single-pane window with area 0.15 m2 to that for a double-pane window with the same area. The glass of a single pane is 4.2 mm thick, and the air space between the two panes of the double-pane window i
> Many opera singers (and some pop singers) have a range of about 2 1 2 octaves or even greater. Suppose a soprano’s range extends from A below middle C (frequency 220 Hz) up to E-flat above high C (frequency 1244 Hz). Although the vocal tract is quite c
> A 250-kg weight is hanging from the ceiling by a thin copper wire. In its fundamental mode, this wire vibrates at the frequency of concert A (440 Hz). You then increase the temperature of the wire by 40°C . (a). By how much will the fundamental frequenc
> You propose a new temperature scale with temperatures given in M. You define 0.0 M to be the normal melting point of mercury and 100.0 M to be the normal boiling point of mercury. (a). What is the normal boiling point of water in M? (b). A temperatur
> Animals in cold climates often depend on two layers of insulation: a layer of body fat (of thermal conductivity 0.20 W/m ∙ K) surrounded by a layer of air trapped inside fur or down. We can model a black bear (Ursus americanus) as a sphere 1.5 m in diame
> In a container of negligible mass, 0.0400 kg of steam at 100 °C and atmospheric pressure is added to 0.200 kg of water at 50.0 °C. (a). If no heat is lost to the surroundings, what is the final temperature of the system? (b). At the final temperature,
> A Styrofoam bucket of negligible mass contains 1.75 kg of water and 0.450 kg of ice. More ice, from a refrigerator at -15.0 °C, is added to the mixture in the bucket, and when thermal equilibrium has been reached, the total mass of ice in the bucket is 0
> A copper calorimeter can with mass 0.446 kg contains 0.0950 kg of ice. The system is initially at 0.0° C. (a). If 0.0350 kg of steam at 100.0 °C and 1.00 atm pressure is added to the can, what is the final temperature of the calorimeter can and its cont
> A thirsty nurse cools a 2.00-L bottle of a soft drink (mostly water) by pouring it into a large aluminum mug of mass 0.257 kg and adding 0.120 kg of ice initially at -15.0 °C. If the soft drink and mug are initially at 20.0 °C, what is the final temperat
> You have 1.50 kg of water at 28.0° C in an insulated container of negligible mass. You add 0.600 kg of ice that is initially at -22.0° C. Assume that no heat exchanges with the surroundings. (a) After thermal equilibrium has been reached, has all of the
> In a household hot-water heating system, water is delivered to the radiators at 70.0° C (158.0 °F) and leaves at 28.0 °C (82.4 °F). The system is to be replaced by a steam system in which steam at atmospheric pressure condenses in the radiators and the c
> The African bombardier beetle (Stenaptinus insignis) can emit a jet of defensive spray from the movable tip of its abdomen (Fig. P17.91). The beetle’s body has reservoirs containing two chemicals; when the beetle is disturbed, these che
> The human vocal tract is a pipe that extends about 17 cm from the lips to the vocal folds (also called “vocal cords”) near the middle of your throat. The vocal folds behave rather like the reed of a clarinet, and the vocal tract acts like a stopped pipe.
> (a) By how much would the body temperature of the bicyclist in Problem 17.89 increase in an hour if he were unable to get rid of the excess heat? (b). Is this temperature increase large enough to be serious? To find out, how high a fever would it be equ
> If the air temperature is the same as the temperature of your skin (about 30°C), your body cannot get rid of heat by transferring it to the air. In that case, it gets rid of the heat by evaporating water (sweat). During bicycling, a typical 70-kg person’
> The molar heat capacity of a certain substance varies with temperature according to the empirical equation How much heat is necessary to change the temperature of 3.00 mol of this substance from 27°C to 227°C? (Hint: Use Eq. (17.
> (a). A typical student listening attentively to a physics lecture has a heat output of 100 W. How much heat energy does a class of 140 physics students release into a lecture hall over the course of a 50-min lecture? (b). Assume that all the heat energy
> A person of mass 70.0 kg is sitting in the bathtub. The bathtub is 190.0 cm by 80.0 cm; before the person got in, the water was 24.0 cm deep. The water is at 37.0° C. Suppose that the water were to cool down spontaneously to form ice at 0.0 °C, and that
> Suppose that a steel hoop could be constructed to fit just around the earth’s equator at 20.0 °C. What would be the thickness of space between the hoop and the earth if the temperature of the hoop were increased by 0.500°C ?
> You cool a 100.0-g slug of red-hot iron (temperature 745° C) by dropping it into an insulated cup of negligible mass containing 85.0 g of water at 20.0 °C. Assuming no heat exchange with the surroundings, (a). what is the final temperature of the water
> Shivering is your body’s way of generating heat to restore its internal temperature to the normal 37 °C, and it produces approximately 290 W of heat power per square meter of body area. A 68-kg, 1.78-m-tall woman has approximately 1.8 m2 of surface area.
> A typical doughnut contains 2.0 g of protein, 17.0 g of carbohydrates, and 7.0 g of fat. Average food energy values are 4.0 kcal/g for protein and carbohydrates and 9.0 kcal/g for fat. (a). During heavy exercise, an average person uses energy at a rate
> A steel ring with a 2.5000-in. inside diameter at 20.0° C is to be warmed and slipped over a brass shaft with a 2.5020-in. outside diameter at 20.0 °C. (a) To what temperature should the ring be warmed? (b) If the ring and the shaft together are cooled
> The fundamental frequency of a pipe that is open at both ends is 524 Hz. (a). How long is this pipe? If one end is now closed, find (b). the wavelength and (c). the frequency of the new fundamental.
> A Foucault pendulum consists of a brass sphere with a diameter of 35.0 cm suspended from a steel cable 10.5 m long (both measurements made at 20.0° C). Due to a design oversight, the swinging sphere clears the floor by a distance of only 2.00 mm when the
> (a) Equation (17.12) gives the stress required to keep the length of a rod constant as its temperature changes. Show that if the length is permitted to change by an amount L when its temperature changes by T, the stress is equal to where F is the tens
> On a cool (4.0 °C) Saturday morning, a pilot fills the fuel tanks of her Pitts S-2C (a two-seat aerobatic airplane) to their full capacity of 106.0 L. Before flying on Sunday morning, when the temperature is again 4.0 °C, she checks the fuel level and fi
> A metal rod that is 30.0 cm long expands by 0.0650 cm when its temperature is raised from 0.0°C to 100.0 °C. A rod of a different metal and of the same length expands by 0.0350 cm for the same rise in temperature. A third rod, also 30.0 cm long, is made
> A surveyor’s 30.0-m steel tape is correct at 20.0 °C. The distance between two points, as measured by this tape on a day when its temperature is 5.00° C, is 25.970 m. What is the true distance between the points?
> You are making pesto for your pasta and have a cylindrical measuring cup 10.0 cm high made of ordinary glass [
> (a) Equation (16.30) can be written as where c is the speed of light in vacuum, 3.00 × 108 m/s. Most objects move much slower than this (v/c is very small), so calculations made with Eq. (16.30) must be done carefully to avoid rounding err
> A long tube contains air at a pressure of 1.00 atm and a temperature of 77.00C. The tube is open at one end and closed at the other by a movable piston. A tuning fork that vibrates with a frequency of 500 Hz is placed near the open end. Resonance is prod
> A long, closed cylindrical tank contains a diatomic gas that is maintained at a uniform temperature that can be varied. When you measure the speed of sound v in the gas as a function of the temperature T of the gas, you obtain these results: (a). Expla
> A turntable 1.50 m in diameter rotates at 75 rpm. Two speakers, each giving off sound of wavelength 31.3 cm, are attached to the rim of the table at opposite ends of a diameter. A listener stands in front of the turntable. (a). What is the greatest beat
> Standing sound waves are produced in a pipe that is 1.20 m long. For the fundamental and first two overtones, determine the locations along the pipe (measured from the left end) of the displacement nodes and the pressure nodes if (a). the pipe is open a
> A police siren of frequency fsiren is attached to a vibrating platform. The platform and siren oscillate up and down in simple harmonic motion with amplitude Ap and frequency fp. (a). Find the maximum and minimum sound frequencies that you would hear at
> Horseshoe bats (genus Rhinolophus) emit sounds from their nostrils and then listen to the frequency of the sound reflected from their prey to determine the prey’s speed. (The “horseshoe” that gives the bat its name is a depression around the nostrils tha
> A 2.00-MHz sound wave travels through a pregnant woman’s abdomen and is reflected from the fetal heart wall of her unborn baby. The heart wall is moving toward the sound receiver as the heart beats. The reflected sound is then mixed with the transmitted
> The sound source of a ship’s sonar system operates at a frequency of 18.0 kHz. The speed of sound in water (assumed to be at a uniform 200C) is 1482 m/s. (a). What is the wavelength of the waves emitted by the source? (b). What is the difference in fre
> A bat flies toward a wall, emitting a steady sound of frequency 1.70 kHz. This bat hears its own sound plus the sound reflected by the wall. How fast should the bat fly in order to hear a beat frequency of 8.00 Hz?
> Two identical loudspeakers are located at points A and B, 2.00 m apart. The loudspeakers are driven by the same amplifier and produce sound waves with a frequency of 784 Hz. Take the speed of sound in air to be 344 m/s. A small microphone is moved out fr
> The frequency of the note F4 is 349 Hz. (a). If an organ pipe is open at one end and closed at the other, what length must it have for its fundamental mode to produce this note at 20.0 C? (b). At what air temperature will the frequency be 370 Hz, corres
> An organ pipe has two successive harmonics with frequencies 1372 and 1764 Hz. (a). Is this an open or a stopped pipe? Explain. (b). What two harmonics are these? (c). What is the length of the pipe?
> A uniform 165-N bar is supported horizontally by two identical wires A and B (Fig. P16.62). A small 185-N cube of lead is placed threefourths of the way from A to B. The wires are each 75.0 cm long and have a mass of 5.50 g. If both of them are simultane
> A person is playing a small flute 10.75 cm long, open at one end and closed at the other, near a taut string having a fundamental frequency of 600.0 Hz. If the speed of sound is 344.0 m/s, for which harmonics of the flute will the string resonate? In eac
> A geodesic dome constructed with an aluminum framework is a nearly perfect hemisphere; its diameter measures 55.0 m on a winter day at a temperature of -15 °C. How much more interior space does the dome have in the summer, when the temperature is 35 °C?
> The sound from a trumpet radiates uniformly in all directions in 20 C air. At a distance of 5.00 m from the trumpet the sound intensity level is 52.0 dB. The frequency is 587 Hz. (a). What is the pressure amplitude at this distance? (b). What is the dis
> A soprano and a bass are singing a duet. While the soprano sings an A-sharp at 932 Hz, the bass sings an A-sharp but three octaves lower. In this concert hall, the density of air is 1.20 kg/m3 and its bulk modulus is 1.42 × 105 Pa. In order for their not
> A vertical cylindrical tank contains 1.80 mol of an ideal gas under a pressure of 0.300 atm at 20.0 0C. The round part of the tank has a radius of 10.0 cm, and the gas is supporting a piston that can move up and down in the cylinder without friction. The
> A balloon of volume 750 m3 is to be filled with hydrogen at atmospheric pressure (1.01 × 105 Pa). (a). If the hydrogen is stored in cylinders with volumes of 1.90 m3 at a gauge pressure of 1.20 × 106 Pa, how many cylinders are required? Assume that the
> A flask with a volume of 1.50 L, provided with a stopcock, contains ethane gas (C2H6) at 300 K and atmospheric pressure (1.013 × 105 Pa). The molar mass of ethane is 30.1 g/mol. The system is warmed to a temperature of 550 K, with the stopcock open to th

