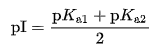Definition of Isoelectric Point
The isoelectric point is a term that is defined as such a pH at which the molecule has no net charge present on it. This means that the molecule is electrically neutral. The charge on the molecule is being influenced by the acidity and basicity of the surrounding environment and due to the gain and loss of protons (H+), the molecule can become more positive or more negative respectively. It is represented by pI, IEP, or pH(I). The isoelectric point is capable of changing the solubility of molecules at a given pH.
Take an example of amino acids which have both acidic and basic functional groups and they impart an overall charge to the protein formed. If the pH is below pI, the protein has a net positive charge whereas if the pH is above the pI, the protein has a net negative charge.
The isoelectric point of an amino acid having one amino and one carbonyl group can be determined by the following formula:
View More Organic Chemistry Definitions
Related Questions of Organic Chemistry
Draw and name four terminal alkynes with the molecular formula C6H10.
Report the result of the following addition to the proper number of
Scorzocreticin (S)-1 was isolated from a plant that is
Write an equation for the proton transfer reaction that occurs when each
87. What is the major product of this reaction?
Propose a stepwise mechanism for the following transformation: /
Identify the sole product of the following reaction: /
Draw the condensation product that is expected when each of the following
Draw a mechanism for each of the following transformations: /
Draw each of the following using condensed formulas and line formulas:
Show All