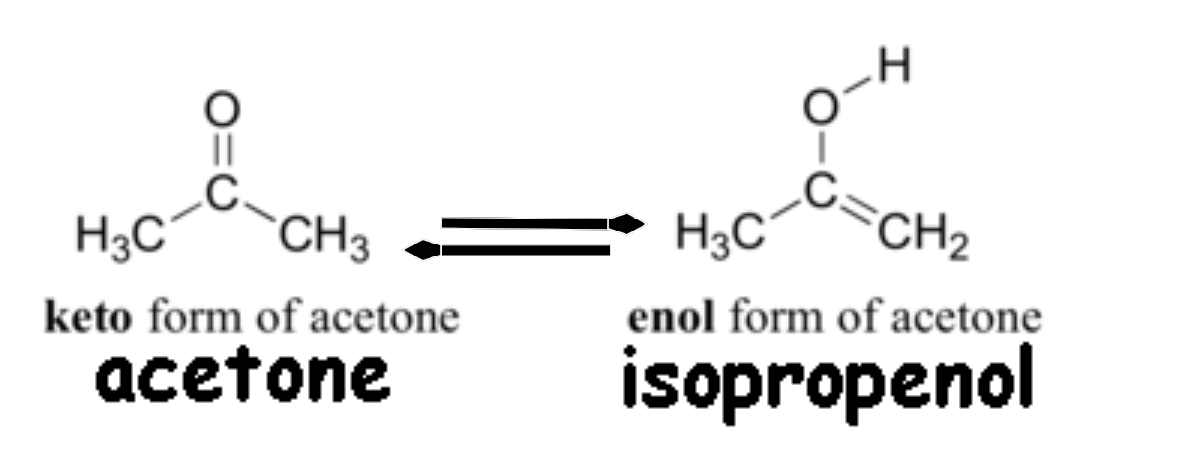
Definition of Keto Enol Tautomerization
Keto-enol-tautomerization is a term that is being used in the field of organic chemistry and it describes the chemical reaction that takes place between a keto compound (a ketone or an aldehyde) and an enol compound (alcohol). Both the groups i.e. keto and enol are known to be tautomers of each other.
The interconversion between the two forms happens by the movement of the α-hydrogen atom (hydrogen linked to the α-carbon) and by the restructuring of the bonding electrons. The acidity of the hydrogen atoms compels the carbonyl group to undergo this process. The equilibrium established between the tautomers is strongly favoured by one of the two isomers and it is quite rapid even under normal conditions.
The enols are formed by two mechanisms. Under the acidic condition, first, the protonation of the carbonyl group occurs and then the enol is formed.
Whereas under basic condition, the enolate is formed first and then the enol is formed.
View More Organic Chemistry Definitions
Related Questions of Organic Chemistry
Draw and name four terminal alkynes with the molecular formula C6H10.
Report the result of the following addition to the proper number of
Scorzocreticin (S)-1 was isolated from a plant that is
Write an equation for the proton transfer reaction that occurs when each
87. What is the major product of this reaction?
Propose a stepwise mechanism for the following transformation: /
Identify the sole product of the following reaction: /
Draw the condensation product that is expected when each of the following
Draw a mechanism for each of the following transformations: /
Draw each of the following using condensed formulas and line formulas:
Show All