Question: Cold water (cp = 4.18 kJ/kg⋅
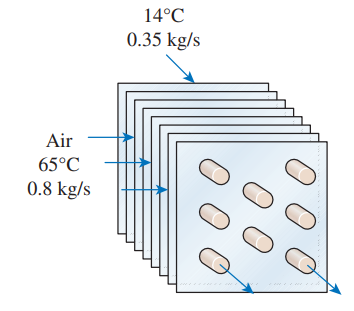
Cold water (cp = 4.18 kJ/kg⋅K) enters a crossflow heat exchanger at 14°C at a rate of 0.35 kg/s where it is heated by hot air (cp = 1.0 kJ/kg⋅K) that enters the heat exchanger at 65°C at a rate of 0.8 kg/s and leaves at 25°C. Determine the maximum outlet temperature of the cold water and the effectiveness of this heat exchanger.
> Propane and methane are commonly used for heating in winter, and the leakage of these fuels, even for short periods, poses a fire danger for homes. Which gas leakage do you think poses a greater risk for fire? Explain.
> What is the difference between mass and molar mass? How are these two related?
> Under what conditions is the ideal-gas assumption suitable for real gases?
> A piston–cylinder device initially contains steam at 3.5 MPa, superheated by 5°C. Now, steam loses heat to the surroundings and the piston moves down, hitting a set of stops, at which point the cylinder contains saturated liq
> The spring-loaded piston–cylinder device shown in Fig. P4–62 is filled with 0.5 kg of water vapor that is initially at 4 MPa and 400°C. Initially, the spring exerts no force against the piston. The spring co
> A piston–cylinder device initially contains 50 L of liquid water at 40°C and 200 kPa. Heat is transferred to the water at constant pressure until all liquid is vaporized. (a) What is the mass of the water? (b) What is the final temperature? (c) Determine
> A rigid tank initially contains 1.4 kg saturated liquid water at 200°C. At this state, 25 percent of the volume is occupied by water and the rest by air. Now heat is supplied to the water until the tank contains saturated vapor only. Determine (a) the vo
> Is it true that water boils at higher temperature at higher pressure? Explain.
> Water is being heated in a vertical piston–cylinder device. The piston has a mass of 40 kg and a cross-sectional area of 150 cm2. If the local atmospheric pressure is 100 kPa, determine the temperature at which the water starts boiling.
> The reactive force developed by a jet engine to push an airplane forward is called thrust, and the thrust developed by the engine of a Boeing 777 is about 85,000 lbf. Express this thrust in N and kgf.
> A piston–cylinder device initially contains 1.4 kg saturated liquid water at 200°C. Now heat is transferred to the water until the volume quadruples and the cylinder contains saturated vapor only. Determine (a) the volume of
> A piston–cylinder device contains 0.6 kg of steam at 300°C and 0.5 MPa. Steam is cooled at constant pressure until one-half of the mass condenses. (a) Show the process on a T-v diagram. (b) Find the final temperature. (c) Determine the volume change.
> 10 kg of R-134a fill a 0.7-m3 weighted piston–cylinder device at a pressure of 200 kPa. The container is now heated until the temperature is 30°C. Determine the initial temperature and final volume of the R-134a.
> One kilogram of water fills a 150-L rigid container at an initial pressure of 2 MPa. The container is then cooled to 40°C. Determine the initial temperature and the final pressure of the water.
> Reconsider Prob. 4–53E. Using appropriate software, investigate the effect of initial pressure on the quality of water at the final state. Let the pressure vary from 100 psia to 300 psia. Plot the quality against initial pressure, and discuss the results
> Superheated water vapor at 180 psia and 500°F is allowed to cool at constant volume until the temperature drops to 250°F. At the final state, determine (a) the pressure, (b) the quality, and (c) the enthalpy. Also, show the process on a T-v diagram with
> A 5-ft3 rigid tank contains a saturated mixture of refrigerant-134a at 50 psia. If the saturated liquid occupies 20 percent of the volume, determine the quality and the total mass of the refrigerant in the tank.
> Reconsider Prob. 4–50. Using appropriate software, investigate the effect of pressure on the total mass of water in the tank. Let the pressure vary from 0.1 MPa to 1 MPa. Plot the total mass of water against pressure, and discuss the re
> A piston–cylinder device contains 0.005 m3 of liquid water and 0.9 m3 of water vapor in equilibrium at 600 kPa. Heat is transferred at constant pressure until the temperature reaches 200°C. (a) What is the initial temperature
> If the pressure of a substance is increased during a boiling process, will the temperature also increase or will it remain constant? Why?
> Solve this system of three equations with three unknowns using appropriate software: x2y − z = 1 x − 3y0.5 + xz = −2 x + y − z = 2
> A rigid tank with a volume of 1.8 m3 contains 40 kg of saturated liquid–vapor mixture of water at 90°C. Now the water is slowly heated. Determine the temperature at which the liquid in the tank is completely vaporized. Also, show the process on a T-v dia
> Water is boiled in a pan covered with a poorly fitting lid at a specified location. Heat is supplied to the pan by a 2-kW resistance heater. The amount of water in the pan is observed to decrease by 1.19 kg in 30 min. If it is estimated that 75 percent o
> Repeat Prob. 4–46 for a location at 2000-m elevation where the standard atmospheric pressure is 79.5 kPa. Data from Prob. 4-46: Water is boiled at 1 atm pressure in a 25-cm-internal-diameter stainless steel pan on an electric range. If it is observed th
> Water is boiled at 1 atm pressure in a 25-cm-internal-diameter stainless steel pan on an electric range. If it is observed that the water level in the pan drops by 10 cm in 45 min, determine the rate of heat transfer to the pan.
> A person cooks a meal in a 30-cm-diameter pot that is covered with a well-fitting lid and lets the food cool to the room temperature of 20°C. The total mass of the food and the pot is 8 kg. Now the person tries to open the pan by lifting the lid up. Assu
> Saturated steam coming off the turbine of a steam power plant at 40°C condenses on the outside of a 3-cm-outer-diameter, 35-m-long tube at a rate of 70 kg/h. Determine the rate of heat transfer from the steam to the cooling water flowing through the pipe
> Water initially at 200 kPa and 300°C is contained in a piston–cylinder device fitted with stops. The water is allowed to cool at constant pressure until it exists as a saturated vapor and the piston rests on the stops. Then t
> 100 kg of R-134a at 200 kPa are contained in a piston–cylinder device whose volume is 12.322 m3. The piston is now moved until the volume is one-half its original size. This is done such that the pressure of the R-134a does not change. Determine the fina
> 10 kg of R-134a at 300 kPa fills a rigid container whose volume is 14 L. Determine the temperature and total enthalpy in the container. The container is now heated until the pressure is 600 kPa. Determine the temperature and total enthalpy when the heati
> Repeat Prob. 4–39 for a location at an elevation of 1500 m where the atmospheric pressure is 84.5 kPa and thus the boiling temperature of water is 95°C. Data from Prob. 4-39: Water is to be boiled at sea level in a 30-cm-dia
> Solve this system of three equations with three unknowns using appropriate software: 2x − y + z = 7 3x2 + 3y = z + 3 xy + 2z = 4
> What is the difference between saturated liquid and compressed liquid?
> Water is to be boiled at sea level in a 30-cm-diameter stainless steel pan placed on top of a 3-kW electric burner. If 60 percent of the heat generated by the burner is transferred to the water during boiling, determine the rate of evaporation of water.
> How much error would one expect in determining the specific enthalpy by applying the incompressible-liquid approximation to water at 3000 psia and 400°F?
> The temperature in a pressure cooker during cooking at sea level is measured to be 250°F. Determine the absolute pressure inside the cooker in psia and in atm. Would you modify your answer if the place were at a higher elevation?
> One kilogram of water vapor at 200 kPa fills the 1.1989-m3 left chamber of a partitioned system shown in Fig. P4–36. The right chamber has twice the volume of the left and is initially evacuated. Determine the pressure of the water afte
> One kilogram of R-134a fills a 0.14-m3 weighted piston–cylinder device at a temperature of 26.4°C. The container is now heated until the temperature is 100°C. Determine the final volume of the R-134a.
> Refrigerant-134a at 200 kPa and 25°C flows through a refrigeration line. Determine its specific volume.
> What is the specific volume of R-134a at 20°C and 700 kPa? What is the internal energy at that state?
> What is the specific volume of water at 5 MPa and 100°C? What would it be if the incompressible liquid approximation were used? Determine the accuracy of this approximation.
> What is the specific internal energy of water at 50 kPa and 200°C?
> Solve this system of two equations with two unknowns using appropriate software: x3 − y2 = 5.9 3xy + y = 3.5
> 10 kg of R-134a fill a 1.115-m3 rigid container at an initial temperature of –30°C. The container is then heated until the pressure is 200 kPa. Determine the final temperature and the initial pressure.
> What is the difference between saturated vapor and superheated vapor?
> Determine the vertical force applied by water on the container.
> A piston–cylinder device contains 0.85 kg of refrigerant 134a at –10°C. The piston that is free to move has a mass of 12 kg and a diameter of 25 cm. The local atmospheric pressure is 88 kPa. Now, heat is tra
> Consider a recuperative crossflow heat exchanger (both fluids unmixed) used in a gas turbine system that carries the exhaust gases at a flow rate of 7.5 kg/s and a temperature of 500°C. The air initially at 30°C and flowing at a rat
> Oil in an engine is being cooled by air in a crossflow heat exchanger, where both fluids are unmixed. Oil (cph = 2047 J/kg⋅K) flowing with a flow rate of 0.026 kg/s enters the heat exchanger at 75°C, while air (cpc = 1007J/kg
> A crossflow air-to-water heat exchanger with an effectiveness of 0.65 is used to heat water (cp = 4180 J/kg⋅K) with hot air (cp = 1010 J/kg⋅K). Water enters the heat exchanger at 20°C at a rate of 4 kg/s, while air enters at 100°C at a rate of 9 kg/s. If
> A crossflow heat exchanger with both fluids unmixed has an overall heat transfer coefficient of 200 W/m2⋅K and a heat transfer surface area of 400 m2. The hot fluid has a heat capacity of 40,000 W/K, while the cold fluid has a heat capacity of 80,000 W/K
> Air (cp = 1005 J/kg⋅K) enters a crossflow heat exchanger at 20°C at a rate of 3 kg/s, where it is heated by a hot water stream (cp = 4190 J/kg⋅K) that enters the heat exchanger at 70°C at a rate of 1 kg/s. Determine the maximum heat transfer rate and the
> In a one-shell and eight-tube-pass heat exchanger, the temperature of water flowing at rate of 50,000 lbm/h is raised from 70°F to 150°F. Hot air (cp = 0.25 Btu/lbm⋅°F) that flows on the tube side enters the heat exchanger at 600°F and exits at 300°F. If
> Cold water (cp = 4180 J/kg⋅K) enters the tubes of a heat exchanger with two shell passes and 23 tube passes at 14°C at a rate of 3 kg/s, while hot oil (cp = 2200 J/kg⋅K) enters the shell at 200°C at the same mass flow rate. The overall heat transfer coef
> A shell-and-tube heat exchanger with two shell passes and eight tube passes is used to heat ethyl alcohol (cp = 2670 J/kg⋅K) in the tubes from 25°C to 70°C at a rate of 2.1 kg/s. The heating is to be done by water (cp = 4190 J/kg⋅K) that enters the shell
> Oil is being cooled from 180°F to 120°F in a one shell and two-tube heat exchanger with an overall heat transfer coefficient of 40 Btu/hâ‹…ft2⋅°F. Water (cpc = 1.0 Btu/lbmâ‹
> One pound-mass of water fills a container whose volume is 2 ft3. The pressure in the container is 100 psia. Calculate the total internal energy and enthalpy in the container.
> How is the thermal resistance due to fouling in a heat exchanger accounted for? How do the fluid velocity and temperature affect fouling?
> Hot oil (cp = 2200 J/kgâ‹…K) is to be cooled by water (cp = 4180 J/kgâ‹…K) in a two-shell-passes and 12-tube-passes heat exchanger. The tubes are thin-walled and are made of copper with a diameter of 1.8 cm. The length of
> In a one-shell and two-tube heat exchanger, cold water with inlet temperature of 20°C is heated by hot water supplied at the inlet at 80°C. The cold and hot water flow rates are 5000 kg/h and 10,000 kg/h, respectively. If the shell-and-tube heat exchange
> A one-shell and two-tube-type heat exchanger has an overall heat transfer coefficient of 300 Btu/h⋅ft2⋅°F. The shell-side fluid has a heat capacity rate of 20,000 Btu/h⋅°F, while the tube-side fluid has a heat capacity rate of 40,000Btu/h⋅°F. The inlet t
> A thin-walled double-pipe counterflow heat exchanger is to be used to cool oil (cp = 0.525 Btu/lbm⋅°F) from 300°F to 105°F at a rate of 5 lbm/s with water (cp = 1.0 Btu/lbm⋅°F) that enters at 70°F at a rate of 3 lbm/s. The diameter of the tube is 5 in, a
> Reconsider Prob. 22–84. Using appropriate software, investigate the effects of the inlet temperature of hot water and the heat transfer coefficient on the rate of heat transfer and the surface area. Let the inlet temperature vary from 6
> Cold water (cp = 4180 J/kg⋅K) leading to a shower enters a thin-walled double-pipe counterflow heat exchanger at 15°C at a rate of 0.25 kg/s and is heated to 45°C by hot water (cp = 4190 J/kg⋅K) t
> Reconsider Prob. 22–82. Using appropriate software, investigate the effects of the mass flow rate of water and the tube length on the outlet temperatures of water and air. Let the mass flow rate vary from 0.05 kg/s to 1.0 kg/s and the tube length from 5
> Water (cp = 4180 J/kg⋅K) is to be heated by solar heated hot air (cp = 1010 J/kg⋅K) in a double pipe counterflow heat exchanger. Air enters the heat exchanger at 90°C at a rate of 0.3 kg/s, while water enters at 22°C at a rate of 0.1 kg/s. The overall he
> Glycerin (cp = 2400 J/kg⋅K) at 20°C and 0.5 kg/s is to be heated by ethylene glycol (cp = 2500 J/kg⋅K) at 60°C and the same mass flow rate in a thin-walled double-pipe parallel-flow heat exchanger. If the overall heat transfer coefficient is 380 W/m2⋅K a
> A 1.8-m3 rigid tank contains steam at 220°C. One-third of the volume is in the liquid phase and the rest is in the vapor form. Determine (a) the pressure of the steam, (b) the quality of the saturated mixture, and (c) the density of the mixtur
> Hot water enters a double-pipe counterflow water-to oil heat exchanger at 190°F and leaves at 100°F. Oil enters at 70°F and leaves at 130°F. Determine which fluid has the smaller heat capacity rate, and calculate the effectiveness of this heat exchanger.
> Consider a double-pipe parallel-flow heat exchanger of length L. The inner and outer diameters of the inner tube are D1 and D2, respectively, and the inner diameter of the outer tube is D3. Explain how you would determine the two heat transfer surface ar
> Consider an oil-to-oil double-pipe heat exchanger whose flow arrangement is not known. The temperature measurements indicate that the cold oil enters at 15°C and leaves at 55°C, while the hot oil enters at 80°C and leaves at 40°C. Do you think this is a
> The radiator in an automobile is a crossflow heat exchanger (UAs = 10 kW/K) that uses air (cp = 1.00 kJ/kg⋅K) to cool the engine-coolant fluid (cp = 4.00 kJ/kg⋅K). The engine fan draws 22°C air through this radiator at a rate of 8 kg/s while the coolant
> Consider a heat exchanger that has an NTU of 0.1. Someone proposes to triple the size of the heat exchanger and thus triple the NTU to 0.3 in order to increase the effectiveness of the heat exchanger and thus save energy. Would you support this proposal?
> Consider a heat exchanger that has an NTU of 4. Someone proposes to double the size of the heat exchanger and thus double the NTU to 8 in order to increase the effectiveness of the heat exchanger and thus save energy. Would you support this proposal?
> How is the NTU of a heat exchanger defined? What does it represent? Is a heat exchanger with a very large NTU (say, 10) necessarily a good one to buy?
> Consider two double-pipe counterflow heat exchangers that are identical except that one is twice as long as the other one. Which heat exchanger is more likely to have a higher effectiveness?
> Consider a shell-and-tube water-to-water heat exchanger with identical mass flow rates for both the hot- and cold-water streams. Now the mass flow rate of the cold water is reduced by half. Will the effectiveness of this heat exchanger increase, decrease
> Consider a double-pipe counterflow heat exchanger. In order to enhance heat transfer, the length of the heat exchanger is now doubled. Do you think its effectiveness will also double?
> Complete this table for refrigerant-134a:
> Under what conditions can a counterflow heat exchanger have an effectiveness of 1? What would your answer be for a parallel-flow heat exchanger?
> Consider a heat exchanger in which both fluids have the same specific heats but different mass flow rates. Which fluid will experience a larger temperature change: the one with the lower or higher mass flow rate?
> Under what conditions is the thermal resistance of the tube in a heat exchanger negligible?
> Can the temperature of the hot fluid drop below the inlet temperature of the cold fluid at any location in a heat exchanger? Explain.
> Explain how you can evaluate the outlet temperatures of the cold and hot fluids in a heat exchanger after its effectiveness is determined.
> For a specified fluid pair, inlet temperatures, and mass flow rates, what kind of heat exchanger will have the highest effectiveness: double-pipe parallel-flow, double-pipe counterflow, crossflow, or multipass shell-and-tube heat exchanger?
> What does the effectiveness of a heat exchanger represent? Can effectiveness be greater than 1? On what factors does the effectiveness of a heat exchanger depend?
> A single-pass crossflow heat exchanger is used to cool jacket water (cp = 1.0 Btu/lbm⋅°F) of a diesel engine from 190°F to 140°F, using air (cp = 0.245 Btu/lbm⋅°F) with an in
> A shell-and-tube heat exchanger with two shell passes and eight tube passes is used to heat ethyl alcohol (cp = 2670 J/kg⋅K) in the tubes from 25°C to 70°C at a rate of 2.1 kg/s. The heating is to be done by water
> Repeat Prob. 22–62 for a mass flow rate of 3 kg/s for water. Data from Prob. 22-62: A shell-and-tube heat exchanger with two shell passes and 12 tube passes is used to heat water (cp = 4180 J/kg⋅K) in the tubes from 20°C to 70°C at a rate of 4.5 kg/s. H
> Complete this table for refrigerant-134a:
> A shell-and-tube heat exchanger with two shell passes and 12 tube passes is used to heat water (cp = 4180 J/kg⋅K) in the tubes from 20°C to 70°C at a rate of 4.5 kg/s. Heat is supplied by hot oil (cp = 2300 J/kg⋅K) that enters the shell side at 170°C at
> Reconsider Prob. 22–60. Using appropriate software, investigate the effect of the mass flow rate of water on the rate of heat transfer and the tube-side surface area. Let the mass flow rate vary from 0.4 kg/s to 2.2 kg/s. Plot the rate of heat transfer a
> A shell-and-tube heat exchanger with two shell passes and 12 tube passes is used to heat water (cp = 4180 J/kg⋅K) with ethylene glycol (cp = 2680 J/kg⋅K). Water enters the tubes at 22°C at a rate of 0.8 kg/s and leaves at 70°C. Ethylene glycol enters the
> What are the heat transfer mechanisms involved during heat transfer in a liquid-to-liquid heat exchanger from the hot to the cold fluid?
> A one-shell-pass and eight-tube-passes heat exchanger is used to heat glycerin (cp = 0.60 Btu/lbm⋅°F) from 80°F to 140°F by hot water (cp = 1.0 Btu/lbm⋅°F) that enters the thin-walled 0.5-in-diameter tubes at 175°F and leaves at 120°F. The total length o
> A shell-and-tube heat exchanger is used for heating 14 kg/s of oil (cp = 2.0 kJ/kg⋅K) from 20°C to 46°C. The heat exchanger has one shell pass and six tube passes. Water enters the shell side at 80°C and leaves at 60°C. The overall heat transfer coeffici
> A test is conducted to determine the overall heat transfer coefficient in a shell-and-tube oil-to water heat exchanger that has 24 tubes of internal diameter 1.2 cm and length 2 m in a single shell. Cold water (cp = 4180 J/kg⋅K) enters the tubes at 20°C
> In an industrial facility, a counterflow double-pipe heat exchanger uses superheated steam at a temperature of 250°C to heat feedwater at 30°C. The superheated steam experiences a temperature drop of 70°C as it exits the
> A performance test is being conducted on a double-pipe counterflow heat exchanger that carries engine oil and water at a flow rate of 2.5 kg/s and 1.75 kg/s, respectively. Since the heat exchanger has been in service for a long time, it is suspected that
> In a textile manufacturing plant, the waste dyeing water (cp = 4295 J/kg⋅K) at 80°C is to be used to preheat fresh water (cp = 4180 J/kg⋅K) at 10°C at the same flow rate in a double-pipe counterfl

