Question: Constr uct an absorption spectr um for
Constr uct an absorption spectr um for a 7.00 × 10-5 M solution of the indicator of Problem 24-19 when measurements are made with 1.00-cm cells and
a)
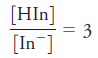
b)
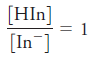
c)
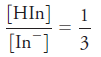
Incicator of Problem 24-19:
Indicator HIn
> Define (a) Atomization. (b) Pressure broadening. (c) Doppler broadening. (d) Aerosol. (e) Plasma. (f) Nebulization. (g) Hollow-cathode lamp. (h) Sputtering. (i) Additive interference. (j) Ionization interference. (k) Chemical interference. (l) Radiation
> Describe the basic differences among atomic emission, atomic absorption, and atomic fluorescence spectroscopy.
> Lead was determined in a brass sample by atomic absorption and the method of standard additions. The original sample was dissolved and diluted to 50.0 mL. This solution was introduced into an AA spectrometer and an absorbance of 0.42 was obtained. To the
> Calculate (a) pI if the cell in Problem 19-17(a) has a potential of -196 mV. (b) pCl if the cell in Problem 19-17(b) has a potential of -0.137 V. (c) pPO4 if the cell in Problem 19-17(c) has a potential of 0.211 V. (d) pCN if the cell in Problem 19-17(d)
> The copper in an aqueous sample was determined by atomic absorption flame spectrometry. First, 10.0 mL of the unknown were pipetted into each of five 50.0-mL volumetric flasks. Various volumes of a standard containing 12.2 ppm Cu were added to the flasks
> The chromium in a series of steel samples was determined by ICP emission spectroscopy. The spectrometer was calibrated with a series of standards containing 0, 2.0, 4.0, 6.0, and 8.0 μg K2Cr2O7 per milliliter. The instrument readings for th
> A 5.00-mL sample of blood was treated with trichloroacetic acid to precipitate proteins. After centrifugation, the resulting solution was brought to pH 3 and extracted with two 5-mL portions of methyl isobutyl ketone containing the lead-complexing agent
> In the atomic absorption determination of uranium, there is a linear relationship between the absorbance at 351.5 nm and concentration from 500 to 2000 ppm of U. At concentrations much lower than 500 ppm, the relationship becomes nonlinear unless about 2
> Discuss the differences that result in ICP atomic emission when the plasma is viewed axially rather than radially.
> Why is the ICP rarely used for atomic absorption measurements?
> Name four characteristics of inductively coupled plasmas that make them suitable for atomic emission spectrometry.
> In flame AA with a hydrogen/oxygen flame, the absorbance for calcium decreases in the presence of large concentrations of phosphate ion. a) Suggest an explanation for this observation. b) Suggest three possible methods for overcoming the potential inte
> Why are the lines from a hollow-cathode lamp generally narrower than the lines emitted by atoms in a flame?
> Why are higher resolution monochromators found in ICP atomic emission spectrometers than in flame atomic absorption spectrometers?
> Generate an equation that relates pAnion to Ecell for each of the cells in Problem 19-17. Cells in Problem 19-17: (a) pI. (b) pCl. (c) pPO4. (d) pCN.
> Why is source modulation used in atomic absorption spectroscopy?
> Why are ionization interferences usually not as severe in the ICP as they are in flames?
> Why do some absorbing compounds show no fluorescence?
> Which compound in each of the following pairs would you expect to have a greater fluorescence quantum yield? Explain. a) b)
> Why are fluorescence methods potentially more sensitive than absorption methods?
> Briefly describe or define (a) Fluorescence. (b) Non-radiative relaxation. (c) Internal conversion. (d) Chemiluminescence. (e) Stokes shift. (f) Secondary absorption. (g) Inner-filter effect. (h) Triplet state.
> The determination in Problem 25-12 was modified to use the standard additions method. In this case, a 2.196-g tablet was dissolved in sufficient 0.10 M HCl to give 1.000 L. Dilution of a 20.00-mL aliquot to 100 mL produced a solution that gave a reading
> Quinine in a 1.664-g antimalarial tablet was dissolved in sufficient 0.10 M HCl to give 500 mL of solution. A 15.00-mL aliquot was then diluted to 100.0 mL with the acid. The fluorescence intensity for the diluted sample at 347.5 nm provided a reading of
> The volumes of a 1.10 ppm standard solution of Zn2+ shown in the following table were pipetted into separatory funnels each containing 5.00 mL of an unknown zinc solution. Each was extracted with three 5-mL aliquots of CCl4 containing an excess of 8-hydr
> The reduced form of nicotinamide adenine dinucleotide (NADH) is an important and highly fluorescent coenzyme. It has an absorption maximum of 340 nm and an emission maximum at 465 nm. Standard solutions of NADH gave the following fluorescence intensities
> Use the shorthand notation to describe a cell consisting of a saturated calomel reference electrode and a silver indicator electrode for the measurement of (a) pI. (b) pCl. (c) pPO4. (d) pCN.
> Why are fluorometers often more useful than spectrofluorometers for quantitative analysis?
> Why are phosphorescence lifetimes much longer than fluorescence lifetimes?
> Describe the components of a filter fluorometer and a spectrofluorometer.
> Explain why fluoresence emission often occurs at a longer wavelength than absorption.
> Describe the characteristics of organic compounds that fluoresce.
> What is(are) advantage(s) of the multiple standard additions method over the single-point standard addition method?
> What experimental variables must be controlled to assure reproducible absorbance data?
> What minimum requirement is needed to obtain reproducible results with a single-beam spectrophotom?
> Describe the differences between the following pairs of terms, and list any particular advantages of one over the other: a) Spectrophotometers and photometers. b) Single-beam and double-beam instruments for absorbance measurements. c) Conventional and
> Predict the shape of photometric titration curves (after correction for volume change) if—at the wavelength selected—the molar absorptivities for the analyte A, the titrant T, and the product P are as follows:
> a) Calculate E0 for the process b) Use the shorthand notation to describe a cell consisting of a Ag/AgCl reference electrode and a lead indicator electrode that could be used for the measurement of pCl. c) Generate an equation that relates the potential
> The equilibrium constant for the conjugate acid-base pair is 8.00 x 10-5. From the additional information a) Calculate the absorbance at 430 nm and 600 nm for the following indicator concentrations: 3.00 × 10-4 M, 2.00 × 10-4
> Estimate the frequencies of the absorption maxima in the IR spectrum of methylene chloride shown in Figure 24F-2. From these frequencies, assign molecular vibrations of methylene chloride to each of the bands. Notice that some of the group frequencies th
> Mercury(II) forms a 1:1 complex with triphenyltetrazolium chloride (TTC) that exhibits an absorption maximum at 255 nm.21 The mercury(II) in a soil sample was extracted into an organic solvent containing an excess of TTC, and the resulting solution was d
> Palladium(II) forms an intensely colored complex at pH 3.5 with arsenazo III at 660 nm.20 A meteorite was pulverized in a ball mill, and the resulting powder was digested with various strong mineral acids. The resulting solution was evaporated to dryness
> The accompanying absorption data were recorded at 390 nm in 1.00-cm cells for a continuous-variations study of the colored product formed between Cd2+ and the complexing reagent R. a) Find the ligand-to-metal ratio in the product. b) Calculate an avera
> The accompanying data were obtained in a slope-ratio investigation of the complex formed between Ni2+ and 1-cyclopentene-1-dithiocarboxylic acid (CDA). The measurements were made at 530 nm in 1.00-cm cells. a) Determine the formula of the complex. Use l
> The sodium salt of 2-quinizarinsulfonic acid (NaQ) forms a complex with Al3+ that absorbs strongly at 560 nm19. The data collected on this system are shown in the accompanying table. a) Find the formula of the complex from the data. In all solutions, cA1
> The method developed in Problem 24-26 was used for the routine determination of iron in 25.0-mL aliquots of ground water. Express the concentration (as ppm Fe) in samples that yielded the accompanying absorbance data (1.00-cm cell). Calculate the relativ
> A standard solution was put through appropriate dilutions to give the concentrations of iron shown in the accompanying table. The iron(II)-1,10,phenanthroline complex was then formed in 25.0-mL aliquots of these solutions, following which each was dilute
> Use the data in Problem 24-24 to calculate the molar concentration of P and Q in each of the following solutions: Data from Problem 24-24: Solutions of P and Q individually obey Beer’s law over a large concentration range. Spectral dat
> a) Calculate E0 for the process (b) Use the shorthand notation to describe a cell consisting of a saturated calomel reference electrode and a silver indicator electrode that could be used to measure pIO3. (c) Develop an equation that relates the potenti
> Solutions of P and Q individually obey Beer’s law over a large concentration range. Spectral data for these species in 1.00-cm cells are a) Plot an absorption spectrum for a solution that is 6.45 × 10-5M in P and 3.21 &Ati
> Several buffer solutions were made 1.00 x 10-4M in the indicator of Problem 24-19. Absorbance data (1.00-cm cells) are Calculate the pH of each solution. Indicator of Problem 24-19: Indicator HIn
> What is the absorbance at 595 nm (1.00-cm cells) of a solution that is 1.25 × 10-4M in the indicator of Problem 24-19 and has a pH of a) 5.30, b) 5.70, c) 6.10? Indicator of Problem 24-19: The indicator HIn
> Calculate the absorbance (1.00-cm cells) at 450 nm of a solution in which the total molar concentration of the indicator described in Problem 24-19 is 8.00 × 10-5M and the pH is a) 4.92, b) 5.46, c) 5.93, d) 6.16.
> The indicator HIn has an acid dissociation constant of 4.80 × 10-6 at ordinary temperatures. The accompanying absorbance data are for 8.00 × 10-5 M solutions of the indicator measured in 1.00-cm cells in strongly acidic and stro
> Molar absorptivity data for the cobalt and nickel complexes with 2,3-quinoxalinedithiol are εCo = 36,400 and εNi = 5520 at 510 nm and εCo = 1240 and εNi = 17,500 at 656 nm. A 0.425-g sample was dissolved and diluted to 50.0 mL. A 25.0-mL aliquot was trea
> A. J. Mukhedkar and N. V. Deshpande (Anal. Chem., 1963, 35, 47, DOI: 10.1021/ac60194a014) report on a simultaneous determination for cobalt and nickel based on absorption by their 8-quinolinol complexes. Molar absorptivities (L mol-1 cm-1 ) are ï
> Iron(III) forms a complex with thiocyanate ion that has the formula Fe(SCN) 2+. The complex has an absorption maximum at 580 nm. A specimen of well water was assayed according to the following scheme. Calculate the concentration of iron in parts per mill
> A 5.24-g petroleum specimen was decomposed by wet ashing and subsequently diluted to 500 mL in a volumetric flask. Cobalt was determined by treating 25.00-mL aliquots of this diluted solution as follows: Assume that the Co(II)/ligand chelate obeys Beer&
> What is the “operational definition of pH”? Why is it used?
> The accompanying data (1.00-cm cells) were obtained for the spectrophotometric titration of 10.00 mL of Pd(II) with 2.44 × 10-4 M Nitroso R (O. W. Rollins and M. M. Oldham, Anal. Chem., 1971, 43, 262, DOI: 10.1021/ac60297a026): Calculate th
> Ethylenediaminetetraacetic acid displaces bismuth(III) from its thiourea complex: Bi(tu)63+ + H2Y2- → BiY- + 6tu + 2H+ where tu is the thiourea molecule, (NH2)2CS. Predict the shape of a photometric titration curve based on this process, given that the
> Iron(III) reacts with thiocyanate ion (SCN) to form the red complex, Fe(SCN)2+. Sketch a photometric titration curve for Fe(III) with thiocyanate ion when a photometer with a green filter is used to collect data. Why is a green filter used?
> Sketch a photometric titration curve for the titration of Sn2+ with MnO-4. What color radiation should be used for this titration? Explain.
> A portable photometer with a linear response to radiation registered 75.5 µA with a blank solution in the light path. Replacement of the blank with an absorbing solution yielded a response of 23.7 µA. Calculate (a) The percent transmittance of the sampl
> A photometer with a linear response to radiation gave a reading of 690 mV with a blank in the light path and 169 mV when the blank was replaced by an absorbing solution. Calculate a) The transmittance and absorbance of the absorbing solution. b) The ex
> The logarithm of the molar absorptivity of phenol in aqueous solution is 4.297 at 211 nm. Calculate the range of phenol concentrations that can be used if the absorbance is to be greater than 0.150 and less than 1.500 with a 1.25-cm cell.
> The logarithm of the molar absorptivity for acetone in ethanol is 2.75 at 366 nm. Calculate the range of acetone concentrations that can be used if the absorbance is to be greater than 0.100 and less than 2.000 with a 1.50-cm cell.
> The molar absorptivity for aqueous solutions of phenol at 211 nm is 5.28 × 103 L cm-1 mol-1. Calculate the permissible range of phenol concentrations if the transmittance is to be less than 85% and greater than 7% when the measurements are made in 1.00-c
> The molar absorptivity for the complex formed between bismuth (III) and thiourea is 9.32 × 103 L cm-1 mol-1 at 470 nm. Calculate the range of permissible concentrations for the complex if the absorbance is to be no less than 0.10 nor greater than 0.90 wh
> Give several advantages of a potentiometric titration over a direct potentiometric measurement.
> Define the term spectral bandpass of a monochromator.
> Describe the differences between the following pairs of terms, and list any particular advantages possessed by one over the other: (a) Solid-state photodiodes and phototubes as detectors for electromagnetic radiation. (b) Phototubes and photomultiplier
> The following data were taken from a diode-array spectrophotometer in an experiment to measure the spectrum of the Co (II)-EDTA complex. The column labeled Psolution is the relative signal obtained with sample solution in the cell after subtraction of th
> An interference filter is to be constructed for isolation of the CS2 absorption band at 4.54 µm. a) If the determination is to be based on first-order interference, how thick should the dielectric layer be (refractive index 1.54)? b) What other wavelen
> Define (a) Transducer. (b) Photocurrent. (c) N-type semiconductor. (d) Majority carrier. (e) Depletion layer. (f) Dynodes in a photomultiplier tube.
> What is the difference between an absorption filter and an interference filter?
> Describe how an absorption photometer and a fluorescence photometer differ from each other.
> Describe the basic design difference between a spectrometer for emission measurements and one for absorption studies.
> What are the differences between a photon detector and a thermal detector?
> Why does a hydrogen lamp produce a continuum rather than a line spectrum in the ultraviolet?
> How does information supplied by a direct potentiometric measurements of pH differ from that obtained from a potentiometric acid-base titration?
> A portable photometer with a linear response to radiation registered a photocurrent of 75.9 μA with a blank solution in the light path. Replacement of the blank with an absorbing solution yielded a response of 23.5 μA. Calculate a) The transmittance of t
> A photometer with a linear response to radiation gave a reading of 625 mV with a blank in the light path and 149 mV when the blank was replaced by an absorbing solution. Calculate a) The percent transmittance and absorbance of the absorbing solution. b)
> What are the major advantages of Fourier transform IR instruments over dispersive IR instruments?
> What non-instrumental variables must be controlled to assure reproducible absorbance data?
> What is the purpose of a) The 0% T adjustment b) The 100% T adjustment of a spectrophotometer?
> What minimum requirement is needed to obtain reproducible results with a single-beam spectrophotometer?
> The relationships described in Problems 23-7 and 23-8 may be of help in solving the following. a) Calculate the wavelength of maximum emission of a tungsten-filament bulb operated at 2870 K and at 3000 K. b) Calculate the total energy output of the bul
> Stefan’s law states that the total energy emitted by a blackbody per unit time and per unit area is Et = αT4 where α is 5.69 × 10-8W/m2 K4. Calculate the total energy output in W>m2 for the blackbodies described in Problem 23-7.
> The Wien displacement law states that the wavelength maximum in micrometers for blackbody radiation is (maxT = 2.90 × 103 Where T is the temperature in kelvins. Calculate the wavelength maximum for a blackbody that has been heated to a) 4000 K, b) 330
> Describe the differences between the following pairs of terms, and list any particular advantages of one over the other: (a) Spectrophotometers and photometers. (b) Spectrographs and spectrometers. (c) Monochromators and polychromators. (d) Single-beam
> What is the source of a) The asymmetry potential in a membrane electrode? b) The boundary potential in a membrane electrode? c) A junction potential in a glass/calomel electrode system? d) The potential of a crystalline-membrane electrode used to determi
> Why is iodine sometimes introduced into a tungsten lamp?
> Why are photomultiplier tubes unsuited for the detection of infrared radiation?
> Why do quantitative and qualitative analyses often require different monochromator slit widths?
> Describe the differences between “real” deviations from Beer’s law and those due to instrumental or chemical factors.
> Identify factors that cause the Beer’s law relationship to be nonlinear.
> What is the relationship between (a) Absorbance and transmittance? (b) Concentration c and molar absorptivity ε?
> In a solution of pH 5.3, the indicator bromocresol purple exhibits a yellow color, but when the pH is 6.0, the indicator solution changes to purple. Discuss why these colors are observed in terms of the wavelength regions and colors absorbed and transmit
> The equilibrium constant for the reaction 2CrO42- + 2H+ ⇌ Cr2O72- + H2O is 4.2 x 1014. The molar absorptivities for the two principal species in a solution of K2CrO7 are Four solutions were prepared by dissolving 4.00 x 10-4, 3.00 x 10
> Nitrite is commonly determined by a colorimetric procedure using a reaction called the Griess reaction. In this reaction, the sample containing nitrite is reacted with sulfanilimide and N-(1-Napthyl) ethylenediamine to form a colored species that absorbs

