Question: Draw the products that are expected for
Draw the products that are expected for each of the following reactions:
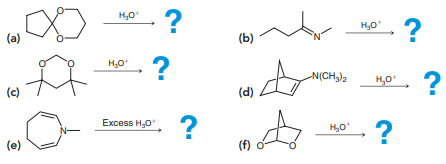
Transcribed Image Text:
? H,0 ? H,0 (а) (b) ? H,0 N(CH,)2 H,0 ? (c) (d) ? Excess H0 ? (е) (f)
> Propose an efficient synthesis for each of the following transformations: (а) (b)
> Using ethanol as your only source of carbon atoms, design a synthesis for the following compound:
> Identify the starting materials needed to make each of the following acetals: x .C OEt (a) (b) (c) o
> Predict the major product(s) for each of the following reactions: CH3 NH2 ? (a) ? 1) PhMgBr 2) H20 (b) ? CH;COH (c) CH,CO,H ? (d) ? -NH2 (-H20) (e)
> Draw a plausible mechanism for each of the following transformations: H,0. (а) H. H,0", H,N. H. (b) [H1 H. но (c) он I-Z
> Identify all of the expected products when the compound below is treated with aqueous acid: ? Excess H,0+ N-
> Predict the major product(s) obtained when each of the following compounds undergoes hydrolysis in the presence of H3O+: (a) (b) (c) (d)
> Identify the reactants that you would use to make each of the following enamines: (a) (b) (с)
> Identify the reactants that you would use to make each of the following imines: (a) (b) (c)
> Using 1-propanol as your only source of carbon, propose an efficient synthesis for each of the following compounds: он H. но. OH (а) (b) (с) н H.
> Glutaraldehyde is a germicidal agent that is sometimes used to sterilize medical equipment too sensitive to be heated in an autoclave. In mildly acidic conditions, glutaraldehyde exists in a cyclic form (below right). Draw a plausible mechanism for this
> Starting with cyclopentanone and using any other reagents of your choosing, identify how you would prepare each of the following compounds: но соон (a) (b) (c) (d)
> Treatment of catechol with formaldehyde in the presence of an acid catalyst produces a compound with the molecular formula C7H6O2. Draw the structure of this product. он HO. Catechol
> Propose a plausible mechanism for the following transformation: но H. EIOH
> Predict the major product(s) from the treatment of acetone with the following: a. [H+], NH3, (−H2O) b. [H+], CH3NH2, (−H2O) c. [H+], excess EtOH, (−H2O) d. [H+], (CH3)2NH, (−H2O) e. [H+], NH2NH2, (−H2O) f. [H+], NH2OH, (−H2O) g. NaBH4, MeOH h. RC
> You are working in a laboratory, and you are given the task of converting cyclopentene into 1,5-pentanediol. Your first thought is simply to perform an ozonolysis followed by reduction with LiAlH4, but your lab is not equipped for an ozonolysis reaction.
> Choose a Grignard reagent and a ketone that can be used to produce each of the following compounds: a. 3-Methyl-3-pentanol b. 1-Ethylcyclohexanol c. Triphenylmethanol d. 5-Phenyl-5-nonanol
> Draw the structure of the alkyl halide needed to prepare each of the following Wittig reagents and then determine which Wittig reagent will be the more difficult to prepare. Explain your choice. Ph. Ph Ph, Ph-P= Ph Ph-P H Ph
> Draw the major product of each Wittig reaction below: Ph Ph. Ph-P= Ph (a) Ph, Ph-P= Ph ? Ph, H. (b) Ph
> For each pair of the following compounds, identify which compound would be expected to react more rapidly with a nucleophile: H. (b) F.C CF3 H,C CH, (a)
> Using cyclopentanone as your starting material and using any other reagents of your choice, propose an efficient synthesis for each of the following compounds: он (а) (Ь) (с)
> Draw all constitutionally isomeric ketones with the molecular formula C6H12O and provide a systematic (IUPAC) name for each isomer.
> Draw all constitutionally isomeric aldehydes with the molecular formula C5H10O and provide a systematic (IUPAC) name for each isomer. Which of these isomers possesses a chiral center?
> Draw all constitutionally isomeric aldehydes with the molecular formula C4H8O and provide a systematic (IUPAC) name for each isomer.
> Provide a systematic (IUPAC) name for the compound below. Be careful: This compound has two chiral centers (can you find them?).
> Draw the structure for each compound below: a. Propanedial b. 4-Phenylbutanal c. (S)-3-Phenylbutanal d. 3,3,5,5-Tetramethyl-4-heptanone e. (R)-3-Hydroxypentanal f. meta-Hydroxyacetophenone g. 2,4,6-Trinitrobenzaldehyde h. Tribromoacetaldehyde i.
> Provide a systematic (IUPAC) name for each of the following compounds: (a) (b) (c) H. (d)
> Compound A has the molecular formula C10H10O and exhibits a strong signal at 1720 cm−1 in its IR spectrum. Treatment with 1,2-ethanedithiol followed by Raney nickel affords the product shown. Identify the structure of compound A. 1)
> The anti-tumor compound maytansine was originally isolated from the Ethiopian shrub Maytenus serrata. Development of a multistep synthesis of maytansine involved compound 1 as a key precursor. Propose an efficient synthesis of 1 starting with acetylene,
> Propose an efficient synthesis for each of the following transformations: он > (a) (b) он он (c) (d) -. (e) (f) EtO OEt
> Predict the major product of each reaction below: H RCO,H .? RCO,H RCO,H (b) (c)
> Identify reagents that can be used to prepare the following compound via a Robinson annulation: H.
> As with aldehydes and ketones, the α position of esters can also be deprotonated. The enolate of ethyl diethoxyacetate was prepared in a synthesis of leustroducsin B, a natural product that has been shown to increase resistance to bacterial
> Identify reagents that you would use to prepare each of the following compounds via a Wittig reaction: Ph (a) (b) Ph (c) EtO
> While developing the synthesis of a natural product, model systems are commonly used to study new reactions. The product formed below was used to prepare such a model compound in the synthesis of the anti-HIV compound didemniserinolipid B. Predict the ma
> The following reaction is from a synthesis of the natural product salinipyrone A. After being synthesized, salinipyrone A was then screened for antitumor activity. Predict the major product of this reaction. Note that the TBS-protected alcohol is stable
> In the following reaction, a Wittig reagent is generated in situ by mixing the phosphonium salt with a strong base, in the presence of an aldehyde (note that ester groups are generally not reactive toward Wittig reagents). Predict the product of this rea
> Predict the major product for each of the following reactions. (EIO),P OE! ? PPhg ? (a) (b) H,C H.
> Identify the reagents necessary to accomplish each of the transformations below: он HO OH (a) он NH2 (b)
> Predict the major product for each of the following reaction sequences: 1) КCN, HCN 2) LIAIH, -? 3) H,0 (a) 1) кCN, HO 2) Н,о", heat (b)
> Identify reagents that can be used to accomplish each of the transformations below: он Ме, он (a) он он (b)
> Predict the major product(s) for each of the following: .? 1) EIMgBr 2) H,0 (a) ? H 1) PhMgBr 2) Н.О (b) 1) PhMgBr 2) H,0 .? (c)
> When 2 moles of benzaldehyde are treated with sodium hydroxide, a reaction occurs in which 1 mole of benzaldehyde is oxidized (giving benzoic acid) while the other mole of benzaldehyde is reduced (giving benzyl alcohol): This reaction, called the Canniz
> Draw a complete mechanism for the following transformation: NaOH, heat
> Predict the major product(s) for each of the following reactions: 1) LIAIH, 2) H,0 ? (a) ? H NABH4, Меон (b) -? 1) LIAIH, 2) H,0 (c) NaBH. :? MEOH (c)
> Draw the structure of the cyclic compound that is produced when acetone is treated with 1,3-propanedithiol in the presence of an acid catalyst. HS SH Acetone 1,3-Propanedithiol
> Predict the major product for each of the following: .? 1) [H"), HS SH 2 Raney Ni (a) `H 1) [H']. HS SH 6) 2) Raney Ni
> As described above, methenamine is hydrolyzed in aqueous acid to produce formaldehyde and ammonia. Draw a mechanism showing formation of one molecule of formaldehyde (the remaining five molecules of formaldehyde are each released via a similar sequence o
> The mechanism for acetal hydrolysis has been heavily investigated. In one study, which explored rates as well as stereochemical aspects, compound 1 was treated with aqueous acid to afford compound 2. Draw the structure of 2, clearly showing the configura
> Predict the product of the following two-step procedure and draw a mechanism for its formation: 1) [H'1 H;N-NH -H,0 2) KOH/H,O, heat :?
> Chiral amines are used to catalyze a host of different enantioselective transformations. For instance, proline (explored further in Chapter 25) is a naturally occurring, chiral amino acid that can be used as a catalyst to transform many simple starting m
> Predict the major product for each of the following reactions: N-H ? [H'] -H,0 (а) -H,0 (b) NH ? [H'] :? [H'] -H,0 -H,0 (с) (d)
> Draw a plausible mechanism for each of the following reactions: 'os*Hl EI,NH [H,So] Me,NH -H,0 (a) (Ь) -H,0
> Tricyclic ring structures, such as compound 6 shown below, are found in a large number of biologically active natural products. These fused rings are challenging to construct, so chemists are continuously working to develop more efficient syntheses. Part
> Identify the reactants that you would use to make each of the following compounds: HO. NH2 (a) (b)
> Predict the product of each of the following reactions: [H') [H'] HO-NH, -H,0 ? H,N-NH, ? (а) (b)
> Pinnatoxin A is a marine natural product isolated from the shellfish Pinna muricata and has demonstrated dangerous toxic effects in humans. Pinnatoxin A has a remarkably intricate structure that includes an iminium group (a protonated imine group). a. A
> Predict the major product for each of the following reactions: [H*) NH3 ? -NH2 -H,0 ? [H'), -H,0 (a) (b) : ? [H'] [H'] NH2 NH2 (с) н -H,0 (d) -H,0
> Draw a plausible mechanism for each of the following transformations: Et [TSOH] MENH, (TSOH) EINH, (а) -H,0 (Ь) -H,0
> Compound A has the molecular formula C8H14O2. Upon treatment with catalytic acid, compound A is converted into the cyclic hemiacetal shown below. Identify the structure of compound A. но, [H'] Compound A
> Draw a plausible mechanism for the following transformation: HO OH [H,SO,]
> Predict the product(s) for each reaction below: OMe ? ? H,0 H,0 OMe (a) (b)
> Propose an efficient synthesis for each of the following transformations: (a) он Ph (b) Ph он (c) H
> The natural product frontalin is a pheromone isolated from the pine beetle Dendroctonus frontalis, a species that accounts for much of the diseased timber found in the northern hemisphere. The following reaction was a step in a synthesis of frontalin: a
> Using a Stork enamine synthesis, show how you might accomplish each of the following transformations: (Ь) (c)
> Draw a plausible mechanism for each of the following transformations: Meo, OMe EtO OEt [H;SOJ excess MeOH [H;SO excess EIOH (a) -H,0 (Ь) -H,0 но OH HO Он 'osH) -H,0 [H,SOJ -H,0 (c) (d)
> For most ketones, hydrate formation is unfavorable, because the equilibrium favors the ketone rather than the hydrate. However, the equilibrium for hydration of hexafluoroacetone favors formation of the hydrate: Provide a plausible explanation for this o
> Draw a mechanism for each of the following reactions: HO HO CI 1) EIMgBr HCI 2) H,0 (a) (b)
> Identify reagents that can be used to achieve each of the following transformations: он OH (a) (b) - (d) H. (f)
> Regular sunscreen use is an important part of keeping your skin healthy by protecting it from ultraviolet radiation, but some chemical agents in sunscreens may be harmful if absorbed into the skin. Research is ongoing to find less harmful alternatives an
> Compounds with two ketone groups are named as alkane diones; for example: The compound above is an artificial flavor added to microwave popcorn and movie- theater popcorn to simulate the butter flavor. Interestingly, this very same compound is also know
> Draw the structure of each of the following compounds: a. (S)-3,3-Dibromo-4-ethylcyclohexanone b. 2,4-Dimethyl-3-pentanone c. (R)-3-Bromobutanal
> Assign a systematic (IUPAC) name to each of the following compounds: (а) Br Br (b) (c) H. (d) - (e)
> In the following reaction, iodine monochloride (ICl) effectively serves as a source of an electrophilic iodonium species, I+. a. Propose a mechanism for the formation of each of the two products, A and B. b. The ratio of A:B in the product mixture is f
> During a synthesis of a potential anticancer agent, 7-hydroxynitidine, the investigators treated bromide 1 with two equivalents of a strong base to form compound 2. Propose a plausible mechanism to account for the following ring-forming transformation:
> In the previous section, we learned how to use diethyl malonate as a starting material in the preparation of substituted carboxylic acids (the malonic ester synthesis). That method employed a step in which the enolate of diethyl malonate attacked an alky
> Compare the indicated bonds (a and b) in the following compound. Bond a has a bond length of 1.45 Ã…, while bond b has a bond length of 1.35 Ã…. Suggest a reason for this difference in bond length for seemingly similar bonds.
> Which of the following is the correct sequence of reactions needed to transform ketone 1 into aldehyde 2, or will all three routes produce the desired product? Explain your choice(s). H Compound 1 Compound 2 Sequence A Sequence B Sequence C (1) (1) (
> Consider the following synthetic sequence: a. Identify reagents that can be used to achieve each of the reactions shown. Note: Reagents have been shown for the conversion of 3 to 4, which involves selective tosylation of a primary alcohol in the presenc
> The following transformation was employed during synthetic studies toward the total synthesis of cyclodidemniserinol trisulfate, found to inhibit HIV-1 integrase. Propose a four-step synthesis to accomplish this transformation. OR EIO" HO Four steps
> Under acid-catalyzed conditions, formaldehyde polymerizes to produce a number of compounds, including metaformaldehyde. Draw a plausible mechanism for this transformation: (H,O*) H H. Metaformaldehyde
> Draw a plausible mechanism for each of the following transformations: H H .N. H + H,C CH, H,0 но (a) OCH, H,0 H. (L) NH,NH, N-H + H. [H,SO] OH HO (d) OCH, осн, (H,SO,] HO. OH (e) OCH, [TSOH] но OH
> Using any compounds of your choosing, identify a method for preparing each of the following compounds. Your only limitation is that the compounds you use can have no more than two carbon atoms. For purposes of counting carbon atoms, you may ignore the ph
> A ketone with the molecular formula C9H18O exhibits only one signal in its 1H NMR spectrum. Provide a systematic (IUPAC) name for this compound.
> A compound with the molecular formula C13H10O produces a strong signal at 1660 cm−1 in its IR spectrum. The 13C NMR spectrum for this compound is shown below. Identify the structure of this compound. Carbon 13 NMR T.villosa -130.0 -
> A compound with the molecular formula C9H10O exhibits a strong signal at 1687 cm−1 in its IR spectrum. The 1H and 13C NMR spectra for this compound are shown below. Identify the structure of this compound. Proton NMR 2 6 5 3 1 Chemi
> Predict the major product of the three following steps and show a mechanism for its formation: 1) Кон ? 2) 3) H,0"
> An aldehyde with the molecular formula C4H6O exhibits an IR signal at 1715 cm−1. a. Propose two possible structures that are consistent with this information. b. Describe how you could use 13C NMR spectroscopy to determine which of the two possible str
> Identify the structures of compounds A to E below: 1) H Br2 Mg A B FeBra 2) H,0 РСС но E OH D [H*).-H,0
> Identify the structures of compounds A to D below and then identify reagents that can be used to convert cyclohexene into compound D in just one step. [H*] NH2NH2 (FH20) H,CrO, KOH /H,O D A B heat
> Using the information provided below, deduce the structures of compounds A, B, C, and D: A D 1) O, 2) DMS 1) EtMgBr 2) H,0 (C,H) (C„H,0) (CH100) B AICI, [H*1, (CHa),NH (-H,0)
> Compound A has the molecular formula C7H14O and reacts with sodium borohydride in methanol to form an alcohol. The 1H NMR spectrum of compound A exhibits only two signals: a doublet (I = 12) and a septet (I = 2). Treating compound A with 1,2-ethanedithio
> Historically, the nitroso group has been known as a strong deactivator, yet an ortho/para-director, in electrophilic aromatic substitution reactions. For example, nitration of nitrosobenzene affords mostly the para-substituted product. a. Rationalize th
> When ortho-bromonitrobenzene is treated with NaOH at elevated temperature, only one product is formed. a. Draw the product. b. Identify the intermediate formed en route to the product. c. Would the reaction occur if the starting compound were metabromo
> Predict the major product of the following reaction: HO,S. So,H ? Dilute HSO.
> When the following compound is treated with Br2 in the presence of a Lewis acid, one product predominates. Determine the structure of that product. Br2 FeBra ?
> The following compound is highly activated but nevertheless undergoes bromination very slowly. Explain.
> Identify the major product formed when each of the following compounds is treated with Et2CuLi followed by mild acid: CN OEt (a) (b) (c)
> When benzene is treated with methyl chloride and aluminum trichloride under conditions that favor trialkylation, one major product is obtained. Draw this product and provide an IUPAC name.
> Starting with benzene and using any other reagents of your choice, design a synthesis for each of the following compounds. In some cases, there may be more than one plausible answer. OMe COOH Br- Br NO2 Rr (a) NO2 (b) COOH (c) NO2 OEt COOH NO, .CI NO

