Question: Each auditing term (or organizational name) in
Each auditing term (or organizational name) in Column 1 below bears a close relationship to a term in Column 2.
Required:
Identify the most closely related terms in Columns 1 and 2. Organize your answer in a two-column format by copying the numbers and terms in Column 1 as given. Then, rearrange the sequence of terms in Column 2 so that each line of your schedule will contain two closely related terms.
Transcribed Image Text:
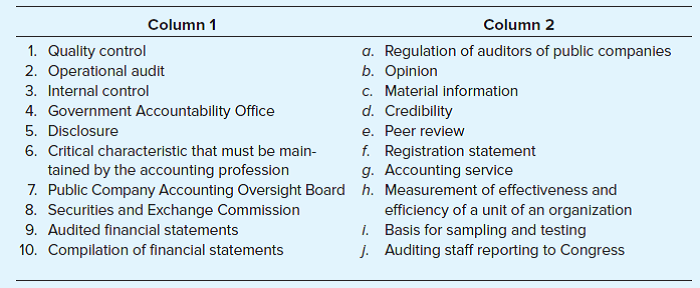
Column 1 Column 2 1. Quality control a. Regulation of auditors of public companies b. Opinion c. Material Information d. Credibility 2. Operational audit 3. Internal control 4. Government Accountability Office 5. Disclosure e. Peer review f. Registration statement g. Accounting service 6. Critical characteristic that must be main- tained by the accounting profession 7. Public Company Accounting Oversight Board h. Measurement of effectiveness and 8. Securities and Exchange Commission 9. Audited financial statements 10. Compilation of financial statements efficiency of a unit of an organization i. Basis for sampling and testing j. Auditing staff reporting to Congress
> What is the International Auditing and Assurance Standards Board? What is the purpose of its pronouncements? Do these pronouncements establish standards that override a member nation’s auditing standards?
> What characteristics make an accounting association an accounting network?
> List three of the more important responsibilities of a partner in a public accounting firm.
> Describe the various levels or positions of accounting personnel in a large public accounting firm.
> Identify and describe the two components of the risk of material misstatement.
> Public accounting firms are sometimes grouped into categories of local firms, regional firms, national firms, and international firms. Explain briefly the characteristics of each. Include in your answer the types of services stressed in each group.
> How does a professional corporation differ from a traditional corporation?
> Will Williams, a college senior, has begun the interviewing process. He has discovered a great variety of organizations in search of “accounting majors.” He finds that various public accounting firms, corporations, the GAO, and the IRS are all interviewi
> What are the advantages of organizing a public accounting firm as a partnership rather than a sole proprietorship?
> Hide-It (HI), a family-owned business based in Tombstone, Arizona, builds custom homes with special features, such as hidden rooms and hidden wall safes. Hide-It has been an audit client for three years. You are about to sign off on a “clean” opinion on
> Houseco, an audit client of Jones, CPA, for the past five years, is a manufacturer of various household products. Approximately four years ago, Houseco developed a better toaster than had been available and sales took off, especially during the most rece
> You are the partner-in-charge of a large metropolitan office of a regional public accounting firm. Two members of your professional staff have come to you to discuss problems that may affect the firm’s independence. Neither of these situations has been s
> The issue of whether the performance of nonattest (consulting) services for audit clients impairs independence of the auditors has been widely debated within the public accounting profession. Restrictions on the performance of consulting are a major aspe
> The following appeared in a brief article in a major business newspaper: A local court is in the process of ruling on whether the public accounting firm of James Willis and Co., CPAs, PC, should be required to pay all or part of $16 million in damages re
> Bart James, a partner in the CPA firm of James and Day, received the following memorandum from John Gray, president of Gray Manufacturing Corporation, an audit client of many years. Dear Bart: I have a new type of engagement for you. You are familiar wit
> What are related party transactions?
> In a discussion between Peters and Ferrel, two auditing students, Peters made the following statement: “A CPA is a professional person who is licensed by the state for the purpose of providing an independent expert opinion on the fairness of financial st
> Feller, the sole owner of a small hardware business, has been told that the business should have its financial statements audited by an independent CPA. Feller, having some bookkeeping experience, has personally prepared the company’s financial statement
> Joe Rezzo, a college student majoring in accounting, helped finance his education with a parttime job maintaining all accounting records for a small business, White Company, located near the campus. Upon graduation, Rezzo passed the CPA examination and j
> Meglow Corporation, a closely held manufacturer of dresses and blouses, sought a loan from Busch Factors. Busch had previously extended $50,000 credit to Meglow but refused to lend any additional money without obtaining copies of Meglow’s audited financi
> Apart from auditing, what other professional services are offered by public accounting firms?
> Risk Capital Limited, a publicly held Delaware corporation, was considering the purchase of a substantial amount of the treasury stock held by Florida Sunshine Corporation, a closely held corporation. Initial discussions with the Florida Sunshine Corpora
> Audit risk should be considered when planning and performing an audit of financial statements in accordance with generally accepted auditing standards. Required: a. Define audit risk. b. Describe its components of inherent risk, control risk, and detect
> Marilyn Terrill is the senior auditor for the audit of Uden Supply Company for the year ended December 31, 20X4. In planning the audit, Marilyn is attempting to develop expectations for planning analytical procedures based on the financial information fo
> Criticize the following working paper that you are reviewing as senior auditor on the December 31 audit of Pratt Company. Pratt Company Cash Per bank $44,874.50v Deposit In transit 5,843.100 Bank charges (2.80y2 Outstanding checks 1,246.40v 3,412.7
> Included in the financial statements are a variety of accounting estimates (e.g., allowance for doubtful accounts, obsolete inventory, warranty liability). Audit procedures should be designed to obtain evidence about the assertions of management related
> Explain the following statement: One contribution of the independent auditor is to lend credibility to financial statements.
> Trend analysis, common-size financial statements, and ratios are presented for the Brody Corporation in Figure 5.4. Assume that you are auditing Brody’s financial statements for the year ended 12/31/X8. You have performed tests of contr
> During your examination of the accounts receivable of Hope Ranch, a new client, you notice that one account is much larger than the rest, and you therefore decide to examine the evidence supporting this customer’s account. Comment on the relative reliabi
> Assume that the auditors find serious weaknesses in the internal control of Oak Canyon, Inc., a producer and distributor of fine wines. Would these internal control weaknesses cause the auditors to rely more or less upon each of the following types of ev
> Mark Williams, CPA, was engaged by Jackson Financial Development Company to audit the financial statements of Apex Construction Company, a small closely held corporation. Williams was told when he was engaged that Jackson Financial needed reliable finan
> The limitations on professional responsibilities of CPAs when they are associated with unaudited financial statements are often misunderstood. These misunderstandings can be reduced substantially if CPAs carefully follow professional pronouncements in th
> How does the role of the SEC differ from that of the AICPA?
> Use the Code of Professional Conduct (available at pub.aicpa.org/codeofconduct) to research each of the circumstances presented in Problem 3-40 and address whether Bell & Greer’s independence is impaired. Provide the section of the Independence Rule and
> Use the Code of Professional Conduct (available at pub.aicpa.org/codeofconduct) to research each of the circumstances presented in Problem 3-39 and address whether Daleiden would violate the Code. Provide the Rule of the Code of Professional Conduct invo
> This problem requires access to the AICPA Code of Professional Conduct at pub.aicpa.org/ codeofconduct. Sandra Singer is the partner in charge of the Minneapolis office of Wellsley and Associates, CPAs. Sandra asked you to research several issues related
> Thomas Gilbert and Susan Bradley formed a professional corporation called “Financial Services Inc.—A Professional Corporation,” each taking 50 percent of the authorized common stock. Gilbert is a CPA and a member of the AICPA. Bradley is a CPCU (Chartere
> When auditing a client’s asset that is valued at fair value, would the auditors expect that asset to be valued at the price to purchase the asset as of the measurement date or the price that would be received to sell it? Explain.
> Roland Company, a retail store, has utilized your services as independent auditor for several years. During the current year, the company opened a new store, and in the course of your annual audit, you verify the cost of the fixtures installed in the new
> Gary Watson, a graduating business student at a small college, is currently interviewing for a job. Gary was invited by both Tilly Manufacturing Co. and Watson Supply Company to travel to a nearby city for an interview. Both companies have offered to pay
> Charles Worthington, the founding and senior partner of a successful and respected public accounting firm, was a highly competent practitioner who always emphasized high professional standards. One of the policies of the firm was that all reports by memb
> After Commuter Airlines was forced into bankruptcy, the company’s stockholders brought suit against Thomas & Ross, the company’s independent auditors. Three independent assumptions concerning this litigation are listed below: a. Commuter Airlines is not
> Cragsmore & Company, a medium-sized partnership of CPAs, was engaged by Marlowe Manufacturing, Inc., a closely held corporation, to audit its financial statements for the year ended December 31, 20X3. Before preparing the audit report, William Cragsmore,
> Match the following definitions (or partial definitions) of the various types of services to the appropriate term. Each term may be used once or not at all. Definition (or Partial Definition) Service a. A government agency authorized to regulate co
> What is meant by a peer review in public accounting?
> Smith & Co., a local Dallas public accounting firm, is incorporated as a professional corporation, with three shareholders, all CPAs. The shareholders have developed a combination of marketing, software, and professional expertise that has allowed them t
> Match the following definitions (or partial definitions) of the various types of services to the appropriate service. Each service may be used once or not at all. Definition (or Partial Definition) Service a. An attest engagement In which the CPAS
> CPAs become involved in a variety of types of engagements. For each of the following statements, indicate whether it relates to an examination (E), review (R), or agreed-upon procedures (A) engagement. If the statement does not relate to examinations, re
> In deciding upon the type of evidence to be gathered in support of a given item on the financial statements, the auditors should not be influenced by the differences in cost of obtaining alternative forms of evidence.” Do you agree? Explain.
> Auditors should be familiar with available professional literature from a variety of sources. Listed next are 11 publications in the fields of auditing and accounting. 1. Statements on Auditing Standards (SASs). 2. The Journal of Accountancy. 3. Regulati
> The role of the auditor in the American economy has changed over the years in response to changes in our economic and political institutions. Consequently, the nature of an audit today is quite different from that of an audit performed in the year 1900.
> Select the best answer for each of the following items and give reasons for your choice. a. Which of the following best describes the relationship between assurance services and attest services? (1) While attest services involve financial data, assurance
> Select the best answer for each of the following questions and explain the reasons for your choice. a. If a CPA performs an audit recklessly, the CPA will be liable to third parties who were unknown and not foreseeable to the CPA for: (1) Strict liabilit
> During the audit of a dealership selling only new luxury automobiles, the auditors calculated the year 2 and year 1 ratios in the table below. Select the most reasonable explanation the controller will provide relating to year 2 for each change. An ex
> Reply to the following questions relating to analytical procedures. a. Performing analytical procedures may help an auditor to: (1) Achieve audit objectives related to a particular assertion. (2) Develop an effective system of quality control. (3) Meet P
> State whether each of the following statements is correct or incorrect concerning audit risk and its components—inherent risk, control risk, and detection risk. a. The risk of material misstatement is composed of the three components of audit risk. b. In
> List two of the important contributions to auditing literature by the AICPA.
> Auditors consider financial statement assertions to identify appropriate audit procedures. For items a through f, match each assertion with the statement that most closely approximates its meaning. Each statement may be used only once. Auditors perfor
> List and briefly describe the three approaches to auditing accounting estimates that are included in a client’s financial statements.
> Enormo Corporation is a large multinational audit client of your CPA firm. One of Enormo’s subsidiaries, Ultro, Ltd., is a successful electronics assembly company that operates in a small Caribbean country. The country in which Ultro operates has very st
> Does it make sense to ask, “If the universe is expanding, what is it expanding into?”
> Does the universe have a center? Explain.
> Is it possible that some parts of the universe contain antimatter whose atoms have nuclei made of antiprotons and antineutrons, surrounded by positrons? How could we detect this condition without actually going there? Can we detect these antiatoms by ide
> Since lead is a stable element, why doesn’t the 238U decay series shown in Fig. 43.7 stop at lead, 214Pb? From Fig. 43.7 43.7 Segrè chart showing the uranium 238U decay series, terminating with the stable nuclide 200Pb. The times
> As stars age, they use up their supply of hydrogen and eventually begin producing energy by a reaction that involves the fusion of three helium nuclei to form a carbon nucleus. Would you expect the interiors of these old stars to be hotter or cooler than
> Heavy, unstable nuclei usually decay by emitting an α or a β particle. Why don’t they usually emit a single proton or neutron?
> The binding energy per nucleon for most nuclides doesn’t vary much (see Fig. 43.2). Is there similar consistency in the atomic energy of atoms, on an “energy per electron” basis? If so, why? If not, w
> What are the six known elements for which Z is a magic number? Discuss what properties these elements have as a consequence of their special values of Z.
> The only two stable nuclides with more protons than neutrons are 1 1
> Why aren’t the masses of all nuclei integer multiples of the mass of a single nucleon?
> In Chapter 15 we represented a standing wave as a superposition of two waves traveling in opposite directions. Can the wave functions for a particle in a box also be thought of as a combination of two traveling waves? Why or why not? What physical interp
> When a large nucleus splits during nuclear fission, the daughter nuclei of the fission fly apart with enormous kinetic energy. Why does this happen?
> In Eq. (43.11), as the total number of nucleons becomes larger, the importance of the second term in the equation decreases relative to that of the first term. Does this make physical sense? Explain. From Eq. (43.11): Z(Z – 1) C3 A!/3 (А — 22)? C4
> Fission reactions occur only for nuclei with large nucleon numbers, while exoergic fusion reactions occur only for nuclei with small nucleon numbers. Why is this?
> The most common radium isotope found on earth, 226Ra, has a half-life of about 1600 years. If the earth was formed well over 109 years ago, why is there any radium left now?
> One problem in radiocarbon dating of biological samples, especially very old ones, is that they can easily be contaminated with modern biological material during the measurement process. What effect would such contamination have on the estimated age? Why
> In Example 43.9 (Section 43.4), the activity of atmospheric carbon before 1900 was given. Discuss why this activity may have changed since 1900. From Example 43.9 EXAMPLE 43.9 RADIOCARBON DATING Then, from Eq. (43.16), Before 1900 the activity per
> In the process of internal conversion, a nucleus decays from an excited state to a ground state by giving the excitation energy directly to an atomic electron rather than emitting a gamma-ray photon. Why can this process also produce x-ray photons?
> Why is the alpha, beta, or gamma decay of an unstable nucleus unaffected by the chemical situation of the atom, such as the nature of the molecule or solid in which it is bound? The chemical situation of the atom can, however, have an effect on the half-
> In a nuclear decay equation, why can we represent an electron as 1 0
> If (_Z^A)Eli represents the initial nuclide, what is the decay process or processes if the final nuclide is
> For a particle in a box, what would the probability distribution function Ψ 2 look like if the particle behaved like a classical (Newtonian) particle? Do the actual probability distributions approach this classical form when n is very large? Explain.
> Compared to α particles with the same energy, β particles can much more easily penetrate through matter. Why is this?
> In the 238U decay series shown in Fig. 43.7, some nuclides in the series are found much more abundantly in nature than others, even though every 238U nucleus goes through every step in the series before finally becoming 206Pb. Why don’t
> Neutrons have a magnetic dipole moment and can undergo spin flips by absorbing electromagnetic radiation. Why, then, are protons rather than neutrons used in MRI of body tissues? (See Fig. 43.1.) From Fig. 43.1 43.1 Magnetic resonance imaging (MRI)
> In what ways do atoms in a diatomic molecule behave as though they were held together by a spring? In what ways is this a poor description of the interaction between the atoms?
> The air you are breathing contains primarily nitrogen (N2) and oxygen (O2). Many of these molecules are in excited rotational energy levels (l = 1, 2, 3, ……), but almost all of them are in the vibrational ground level (n = 0). Explain this difference bet
> Various organic molecules have been discovered in interstellar space. Why were these discoveries made with radio telescopes rather than optical telescopes?
> Discuss the differences between the rotational and vibrational energy levels of the deuterium (“heavy hydrogen”) molecule D2 and those of the ordinary hydrogen molecule H2. A deuterium atom has twice the mass of an ordinary hydrogen atom.
> Many radioactive decays occur within a sequence of decays—for example, 92 234
> Radioisotopes are used in a variety of manufacturing and testing techniques. Wear measurements can be made using the following method. An automobile engine is produced using piston rings with a total mass of 100 g, which includes 9.4 µCi of 59Fe whose ha
> The H2+ molecule consists of two hydrogen nuclei and a single electron. What kind of molecular bond do you think holds this molecule together? Explain.
> If Ψ is normalized, what is the physical significance of the area under a graph of Ψ 2 versus x between x1 and x2? What is the total area under the graph of Ψ 2 when all x are included? Explain.
> Consider a simple model of the helium atom in which two electrons, each with mass m, move around the nucleus (charge +2e) in the same circular orbit. Each electron has orbital angular momentum ħ (that is, the orbit is the smallest-radius Bohr
> Each of 2N electrons (mass m) is free to move along the x-axis. The potential-energy function for each electron is U(x)= 1/2 k′x2, where k′ is a positive constant. The electric and magnetic interactions between electrons can be ignored. Use the exclusion
> How could you make compensated silicon that has twice as many acceptors as donors?
> Protons, neutrons, and many other particles are made of more fundamental particles called quarks and antiquarks (the antimatter equivalent of quarks). A quark and an antiquark can form a bound state with a variety of different energy levels, each of whic
> The WKB approximation (see Challenge Problem 40.64) can be used to calculate the energy levels for a harmonic oscillator. In this approximation, the energy levels are the solutions to the equation. Here E is the energy, U(x) is the potential-energy fun
> It can be a challenge to solve the Schrödinger equation for the bound-state energy levels of an arbitrary potential well. An alternative approach that can yield good approximate results for the energy levels is the WKB approximation (named f
> There are several methods for removing electrons from the surface of a semiconductor. Can holes be removed from the surface? Explain.

