Question: Neutrons have a magnetic dipole moment and
Neutrons have a magnetic dipole moment and can undergo spin flips by absorbing electromagnetic radiation. Why, then, are protons rather than neutrons used in MRI of body tissues? (See Fig. 43.1.)
From Fig. 43.1
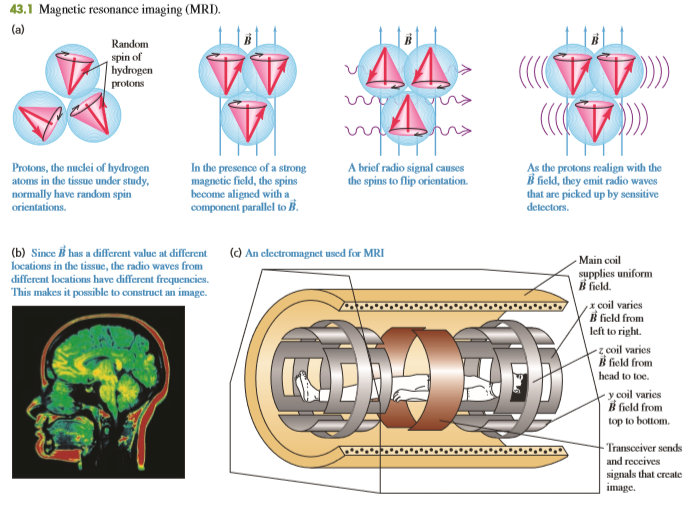
Transcribed Image Text:
43.1 Magnetic resonance imaging (MRI). (a) Random spin of hydrogen protons AA- Protons, the nuclei of hydrogen atoms in the tissue under study, normally have random spin In the presence of a strong magnetic field, the spins become aligned with a component parallel to B. A brief radio signal causes the spins to flip orientation. As the protons realign with the B field, they emit radio waves that are picked up by sensitive detectors. orientations. (b) Since has a different valuc at different locations in the tissue, the radio waves from () An electromagnet used for MRI Main coil different locations have different frequencies. This makes it possible to construct an image. supplies uniform B field. x coil varies B field from left to right. z coil varies B field from head to toe. y coil varies B field from top to bottom. Transceiver sends and reccives signals that create image.
> Thomas Gilbert and Susan Bradley formed a professional corporation called “Financial Services Inc.—A Professional Corporation,” each taking 50 percent of the authorized common stock. Gilbert is a CPA and a member of the AICPA. Bradley is a CPCU (Chartere
> When auditing a client’s asset that is valued at fair value, would the auditors expect that asset to be valued at the price to purchase the asset as of the measurement date or the price that would be received to sell it? Explain.
> Roland Company, a retail store, has utilized your services as independent auditor for several years. During the current year, the company opened a new store, and in the course of your annual audit, you verify the cost of the fixtures installed in the new
> Gary Watson, a graduating business student at a small college, is currently interviewing for a job. Gary was invited by both Tilly Manufacturing Co. and Watson Supply Company to travel to a nearby city for an interview. Both companies have offered to pay
> Charles Worthington, the founding and senior partner of a successful and respected public accounting firm, was a highly competent practitioner who always emphasized high professional standards. One of the policies of the firm was that all reports by memb
> After Commuter Airlines was forced into bankruptcy, the company’s stockholders brought suit against Thomas & Ross, the company’s independent auditors. Three independent assumptions concerning this litigation are listed below: a. Commuter Airlines is not
> Cragsmore & Company, a medium-sized partnership of CPAs, was engaged by Marlowe Manufacturing, Inc., a closely held corporation, to audit its financial statements for the year ended December 31, 20X3. Before preparing the audit report, William Cragsmore,
> Match the following definitions (or partial definitions) of the various types of services to the appropriate term. Each term may be used once or not at all. Definition (or Partial Definition) Service a. A government agency authorized to regulate co
> What is meant by a peer review in public accounting?
> Smith & Co., a local Dallas public accounting firm, is incorporated as a professional corporation, with three shareholders, all CPAs. The shareholders have developed a combination of marketing, software, and professional expertise that has allowed them t
> Match the following definitions (or partial definitions) of the various types of services to the appropriate service. Each service may be used once or not at all. Definition (or Partial Definition) Service a. An attest engagement In which the CPAS
> CPAs become involved in a variety of types of engagements. For each of the following statements, indicate whether it relates to an examination (E), review (R), or agreed-upon procedures (A) engagement. If the statement does not relate to examinations, re
> In deciding upon the type of evidence to be gathered in support of a given item on the financial statements, the auditors should not be influenced by the differences in cost of obtaining alternative forms of evidence.” Do you agree? Explain.
> Each auditing term (or organizational name) in Column 1 below bears a close relationship to a term in Column 2. Required: Identify the most closely related terms in Columns 1 and 2. Organize your answer in a two-column format by copying the numbers an
> Auditors should be familiar with available professional literature from a variety of sources. Listed next are 11 publications in the fields of auditing and accounting. 1. Statements on Auditing Standards (SASs). 2. The Journal of Accountancy. 3. Regulati
> The role of the auditor in the American economy has changed over the years in response to changes in our economic and political institutions. Consequently, the nature of an audit today is quite different from that of an audit performed in the year 1900.
> Select the best answer for each of the following items and give reasons for your choice. a. Which of the following best describes the relationship between assurance services and attest services? (1) While attest services involve financial data, assurance
> Select the best answer for each of the following questions and explain the reasons for your choice. a. If a CPA performs an audit recklessly, the CPA will be liable to third parties who were unknown and not foreseeable to the CPA for: (1) Strict liabilit
> During the audit of a dealership selling only new luxury automobiles, the auditors calculated the year 2 and year 1 ratios in the table below. Select the most reasonable explanation the controller will provide relating to year 2 for each change. An ex
> Reply to the following questions relating to analytical procedures. a. Performing analytical procedures may help an auditor to: (1) Achieve audit objectives related to a particular assertion. (2) Develop an effective system of quality control. (3) Meet P
> State whether each of the following statements is correct or incorrect concerning audit risk and its components—inherent risk, control risk, and detection risk. a. The risk of material misstatement is composed of the three components of audit risk. b. In
> List two of the important contributions to auditing literature by the AICPA.
> Auditors consider financial statement assertions to identify appropriate audit procedures. For items a through f, match each assertion with the statement that most closely approximates its meaning. Each statement may be used only once. Auditors perfor
> List and briefly describe the three approaches to auditing accounting estimates that are included in a client’s financial statements.
> Enormo Corporation is a large multinational audit client of your CPA firm. One of Enormo’s subsidiaries, Ultro, Ltd., is a successful electronics assembly company that operates in a small Caribbean country. The country in which Ultro operates has very st
> Does it make sense to ask, “If the universe is expanding, what is it expanding into?”
> Does the universe have a center? Explain.
> Is it possible that some parts of the universe contain antimatter whose atoms have nuclei made of antiprotons and antineutrons, surrounded by positrons? How could we detect this condition without actually going there? Can we detect these antiatoms by ide
> Since lead is a stable element, why doesn’t the 238U decay series shown in Fig. 43.7 stop at lead, 214Pb? From Fig. 43.7 43.7 Segrè chart showing the uranium 238U decay series, terminating with the stable nuclide 200Pb. The times
> As stars age, they use up their supply of hydrogen and eventually begin producing energy by a reaction that involves the fusion of three helium nuclei to form a carbon nucleus. Would you expect the interiors of these old stars to be hotter or cooler than
> Heavy, unstable nuclei usually decay by emitting an α or a β particle. Why don’t they usually emit a single proton or neutron?
> The binding energy per nucleon for most nuclides doesn’t vary much (see Fig. 43.2). Is there similar consistency in the atomic energy of atoms, on an “energy per electron” basis? If so, why? If not, w
> What are the six known elements for which Z is a magic number? Discuss what properties these elements have as a consequence of their special values of Z.
> The only two stable nuclides with more protons than neutrons are 1 1
> Why aren’t the masses of all nuclei integer multiples of the mass of a single nucleon?
> In Chapter 15 we represented a standing wave as a superposition of two waves traveling in opposite directions. Can the wave functions for a particle in a box also be thought of as a combination of two traveling waves? Why or why not? What physical interp
> When a large nucleus splits during nuclear fission, the daughter nuclei of the fission fly apart with enormous kinetic energy. Why does this happen?
> In Eq. (43.11), as the total number of nucleons becomes larger, the importance of the second term in the equation decreases relative to that of the first term. Does this make physical sense? Explain. From Eq. (43.11): Z(Z – 1) C3 A!/3 (А — 22)? C4
> Fission reactions occur only for nuclei with large nucleon numbers, while exoergic fusion reactions occur only for nuclei with small nucleon numbers. Why is this?
> The most common radium isotope found on earth, 226Ra, has a half-life of about 1600 years. If the earth was formed well over 109 years ago, why is there any radium left now?
> One problem in radiocarbon dating of biological samples, especially very old ones, is that they can easily be contaminated with modern biological material during the measurement process. What effect would such contamination have on the estimated age? Why
> In Example 43.9 (Section 43.4), the activity of atmospheric carbon before 1900 was given. Discuss why this activity may have changed since 1900. From Example 43.9 EXAMPLE 43.9 RADIOCARBON DATING Then, from Eq. (43.16), Before 1900 the activity per
> In the process of internal conversion, a nucleus decays from an excited state to a ground state by giving the excitation energy directly to an atomic electron rather than emitting a gamma-ray photon. Why can this process also produce x-ray photons?
> Why is the alpha, beta, or gamma decay of an unstable nucleus unaffected by the chemical situation of the atom, such as the nature of the molecule or solid in which it is bound? The chemical situation of the atom can, however, have an effect on the half-
> In a nuclear decay equation, why can we represent an electron as 1 0
> If (_Z^A)Eli represents the initial nuclide, what is the decay process or processes if the final nuclide is
> For a particle in a box, what would the probability distribution function Ψ 2 look like if the particle behaved like a classical (Newtonian) particle? Do the actual probability distributions approach this classical form when n is very large? Explain.
> Compared to α particles with the same energy, β particles can much more easily penetrate through matter. Why is this?
> In the 238U decay series shown in Fig. 43.7, some nuclides in the series are found much more abundantly in nature than others, even though every 238U nucleus goes through every step in the series before finally becoming 206Pb. Why don’t
> In what ways do atoms in a diatomic molecule behave as though they were held together by a spring? In what ways is this a poor description of the interaction between the atoms?
> The air you are breathing contains primarily nitrogen (N2) and oxygen (O2). Many of these molecules are in excited rotational energy levels (l = 1, 2, 3, ……), but almost all of them are in the vibrational ground level (n = 0). Explain this difference bet
> Various organic molecules have been discovered in interstellar space. Why were these discoveries made with radio telescopes rather than optical telescopes?
> Discuss the differences between the rotational and vibrational energy levels of the deuterium (“heavy hydrogen”) molecule D2 and those of the ordinary hydrogen molecule H2. A deuterium atom has twice the mass of an ordinary hydrogen atom.
> Many radioactive decays occur within a sequence of decays—for example, 92 234
> Radioisotopes are used in a variety of manufacturing and testing techniques. Wear measurements can be made using the following method. An automobile engine is produced using piston rings with a total mass of 100 g, which includes 9.4 µCi of 59Fe whose ha
> The H2+ molecule consists of two hydrogen nuclei and a single electron. What kind of molecular bond do you think holds this molecule together? Explain.
> If Ψ is normalized, what is the physical significance of the area under a graph of Ψ 2 versus x between x1 and x2? What is the total area under the graph of Ψ 2 when all x are included? Explain.
> Consider a simple model of the helium atom in which two electrons, each with mass m, move around the nucleus (charge +2e) in the same circular orbit. Each electron has orbital angular momentum ħ (that is, the orbit is the smallest-radius Bohr
> Each of 2N electrons (mass m) is free to move along the x-axis. The potential-energy function for each electron is U(x)= 1/2 k′x2, where k′ is a positive constant. The electric and magnetic interactions between electrons can be ignored. Use the exclusion
> How could you make compensated silicon that has twice as many acceptors as donors?
> Protons, neutrons, and many other particles are made of more fundamental particles called quarks and antiquarks (the antimatter equivalent of quarks). A quark and an antiquark can form a bound state with a variety of different energy levels, each of whic
> The WKB approximation (see Challenge Problem 40.64) can be used to calculate the energy levels for a harmonic oscillator. In this approximation, the energy levels are the solutions to the equation. Here E is the energy, U(x) is the potential-energy fun
> It can be a challenge to solve the Schrödinger equation for the bound-state energy levels of an arbitrary potential well. An alternative approach that can yield good approximate results for the energy levels is the WKB approximation (named f
> There are several methods for removing electrons from the surface of a semiconductor. Can holes be removed from the surface? Explain.
> What is the essential characteristic for an element to serve as a donor impurity in a semiconductor such as Si or Ge? For it to serve as an acceptor impurity? Explain.
> Why are materials that are good thermal conductors also good electrical conductors? What kinds of problems does this pose for the design of appliances such as clothes irons and electric heaters? Are there materials that do not follow this general rule?
> The assumptions of the free-electron model of metals may seem contrary to reason, since electrons exert powerful electric forces on each other. Give some reasons why these assumptions actually make physical sense.
> For the particle in a box, we chose k = nπ/L with n = 1, 2, 3, …………. to fit the boundary condition that Ψ = 0 at x = L. However, n = 0, -1, -2, -3, …… also satisfy that boundary condition. Why didn’t we also choose those values of n?
> An isolated zinc atom has a ground-state electron configuration of filled 1s, 2s, 2p, 3s, 3p, and 4s subshells. How can zinc be a conductor if its valence subshell is full?
> Use the band model to explain how it is possible for some materials to undergo a semiconductor-to-metal transition as the temperature or pressure varies.
> Speeds of molecules in a gas vary with temperature, whereas speeds of electrons in the conduction band of a metal are nearly independent of temperature. Why are these behaviors so different?
> Consider a collision in which a stationary particle with mass M is bombarded by a particle with mass m, speed v0, and total energy (including rest energy) Em. a. Use the Lorentz transformation to write the velocities vm and vM of particles m and M in te
> What factors determine whether a material is a conductor of electricity or an insulator? Explain.
> When the pressure p on a material increases by an amount ∆p, the volume of the material will change from V to V + ∆V, where ∆V is negative. The bulk modulus B of the material is defined to be the rati
> Consider a system of N free electrons within a volume V. Even at absolute zero, such a system exerts a pressure p on its surroundings due to the motion of the electrons. To calculate this pressure, imagine that the volume increases by a small amount dV.
> An electron in a hydrogen atom is in an s level, and the atom is in a magnetic field B = B
> In the ground state of the helium atom one electron must have “spin down” and the other “spin up.” Why?
> In the Stern–Gerlach experiment, why is it essential for the magnetic field to be inhomogeneous (that is, nonuniform)?
> If a particle is in a stationary state, does that mean that the particle is not moving? If a particle moves in empty space with constant momentum p and hence constant energy E = p2/2m, is it in a stationary state? Explain your answers.
> a. If two electrons in hydrogen atoms have the same principal quantum number, can they have different orbital angular momenta? How? b. If two electrons in hydrogen atoms have the same orbital quantum number, can they have different principal quantum num
> The Stern–Gerlach experiment is always performed with beams of neutral atoms. Wouldn’t it be easier to form beams using ionized atoms? Why won’t this work?
> Why is the analysis of the helium atom much more complex than that of the hydrogen atom, either in a Bohr type of model or using the Schrödinger equation?
> For a body orbiting the sun, such as a planet, comet, or asteroid, is there any restriction on the z-component of its orbital angular momentum such as there is with the z-component of the electron’s orbital angular momentum in hydrogen? Explain.
> Repeat Discussion Question Q41.24 for the wave function Ψ(r1, r2) = Ψα(r1)Ψα(r2). From Q41.24: A system of two electrons has the wave function Ψ(r1, r2)=(1/ 2 )[Ψa(r1) Ψb(r2)- Ψb(r1)Ψa(r2)], where ca is a normalized wave function for a state with Sz =
> The binding energy of a potassium chloride molecule (KCl) is 4.43 eV. The ionization energy of a potassium atom is 4.3 eV, and the electron affinity of chlorine is 3.6 eV. Use these data to estimate the equilibrium separation between the two atoms in the
> a. The equilibrium separation of the two nuclei in an NaCl molecule is 0.24 nm. If the molecule is modeled as charges +e and -e separated by 0.24 nm, what is the electric dipole moment of the molecule (see Section 21.7)? b. The measured electric dipole
> When a diatomic molecule undergoes a transition from the l = 2 to the l = 1 rotational state, a photon with wavelength 54.3 µm is emitted. What is the moment of inertia of the molecule for an axis through its center of mass and perpendicular to the line
> A hypothetical diatomic molecule of oxygen (mass = 2.656 * 10-26 kg) and hydrogen (mass = 1.67 * 10-27 kg) emits a photon of wavelength 2.39 µm when it makes a transition from one vibrational state to the next lower state. If we model this molecule as tw
> You have entered a graduate program in particle physics and are learning about the use of symmetry. You begin by repeating the analysis that led to the prediction of the Ω- particle. Nine of the spin-3/2 baryons are four ∆ particles, each with mass 1232
> The decay products from the decay of shortlived unstable particles can provide evidence that these particles have been produced in a collision experiment. As an initial step in designing an experiment to detect short-lived hadrons, you make a literature
> While tuning up a medical cyclotron for use in isotope production, you obtain the data given in the table. B is the uniform magnetic field in the cyclotron, and Kmax is the maximum kinetic energy of the particle being accelerated, which is a proton. Th
> A system of two electrons has the wave function Ψ(r1, r2)=(1/ 2 )[Ψa(r1) Ψb(r2)- Ψb(r1)Ψa(r2)], where ca is a normalized wave function for a state with Sz = + 1/2 ħ and Ψb is a normalized wave function for a state with Sz = - 1/2 ħ. a. If Sz for electr
> The K0 meson has rest energy 497.7 MeV. A K0 meson moving in the +x-direction with kinetic energy 225 MeV decays into a π+ and a π-, which move off at equal angles above and below the +x-axis. Calculate the kinetic energy of the π+ and the angle it makes
> A Σ- particle moving in the +x-direction with kinetic energy 180 MeV decays into a π- and a neutron. The π- moves in the +y-direction. What is the kinetic energy of the neutron, and what is the direction of its velocity? Use relativistic expressions for
> A Ξ- particle at rest decays to a Λ0 and a π-. a. Find the total kinetic energy of the decay products. b. What fraction of the energy is carried off by each particle? (Use relativistic expressions for momentum and energy.)
> The densities of ordinary matter and dark matter have decreased as the universe has expanded, since the same amount of mass occupies an ever-increasing volume. Yet observations suggest that the density of dark energy has remained constant over the entire
> A ϕ meson (see Problem 44.45) at rest decays via ϕ → K+ + K-. It has strangeness 0. a. Find the kinetic energy of the K+ meson. (Assume that the two decay products share kinetic energy equally, since their masses are equal.) b. Suggest a reason the dec
> One proposed proton decay is p+ → e+ + π0, which violates both baryon and lepton number conservation, so the proton lifetime is expected to be very long. Suppose the proton half-life were 1.0 * 1018 y. a. Calculate the energy deposited per kilogram of b
> Estimate the energy width (energy uncertainty) of the ψ if its mean lifetime is 7.6 * 10-21 s. What fraction is this of its rest energy?
> The ϕ meson has mass 1019.4 MeV/c2 and a measured energy width of 4.4 MeV/c2. Using the uncertainty principle, estimate the lifetime of the f meson.

