Question: Give the conjugate acid for each compound
Give the conjugate acid for each compound below.
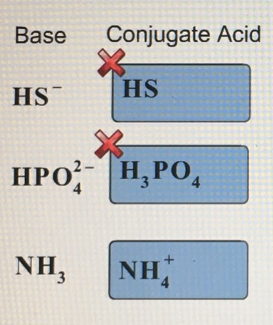
Transcribed Image Text:
Base Conjugate Acid HS HS НРОН, РО, NH, NHA
> Give the shape of CH4 and whether it is polar or nonpolar.
> Identify the following compounds as polar covalent, nonpolar covalent, or ionic H2O CO2 NaI NH3 CH4 A. polar covalent B. nonpolar covalent C. ionic
> Classify each of the molecules given below as polar or nonpolar. SF NH, CIF: XeFa SF PFs BrCls XeF. H20 CS: So, co, CH. Polar Nonpolar
> Complete the following table for XeF4. Activity 1 Lewis Dot Structures Chemical Formula Dot Diagrams Electrons Central Atom Lewis Structure
> Determine whether each molecule is polar or nonpolar. HI CH4 H202 Br2 1. polar NH2OH 2 nonpolar HOCI ICI CH-Cl2 NH3
> 14. Draw Lewis dot structures for the following. Be sure to show all valence electrons as dots or lines a. CF4 b. N2H4 c. OCl2 d. CaCl2 15. Draw the Lewis structure for CH4. What is the electron group/parent structure geometry? What is the molecul
> A. Is CSe2 polar or nonpolar? B. Is CH4 polar or nonpolar? C. Is BrCl3 polar or nonpolar?
> Review VSEPR as needed and determine the molecular geometry of each of the following. Review symmetry elements and determine if each has a mirror plane (σ) (which type), rotation axis (Cn), multiple rotation axes, inversion (i) and use the point group ch
> Describe all the main features of the following theories and explain what useful information we gain from them. How do the theories help describe molecules/ions? What are the pros and cons of the theories? Use a list or chart. a. Lewis Structures b. Va
> Use the data given here to calculate the values of ΔGorxn at 25oC for the reaction described by the equation If ΔHorxn and ΔSorxn are both posi
> Draw the lewis structure with any resonance structures, formal charge calculation, VSEPR: electron geometry and bond angle about, molecular geometry and bond angle about atom same or different than VSEPR? PLEASE BE LEGIBLE AND SHOW ALL WORK! DUE: Fer
> 1. What is the main idea behind VSEPR theory? 2. For each of the following compounds, determine the molecular shapes and bond angles for the central atom. Draw the Lewis dot formula to begin with and use the VESPR Molecular Shape Chart to help you. Is t
> Predicting and Drawing Shapes of Molecules Complete the following chart: Molecular Lewis diagram Name of VSEPR shape Dipole yes/no Formula SeFs SiF. NF3 ÇINO H;S NHạ+ HCN NO,
> For the following molecules, make a chart and fill in the information for the following: How hybridization Compound formula Lewis Structure- VSEPR Drawing Molecular Geometry Electron show all Domain occurred resonance Geometry structures, show formal
> a. What is the formula for ammonium acetate? b. What is the formula for ammonium? c. What is the formula for ammonium chloride?
> 4. What are lone pairs in the VSEPR theory? 5. To determine the shape of a molecule using a VSEPR theory chart, what two things do you need to know. 6. Draw the Lewis dot structure (on back) and complete the table for the following compounds. shape
> Fill in the blank spaces in the following chart. Molecule Lewis structure Electron Bond Molecular domain Angles geometry geometry (shape) (VSEPR) XeF2 -Xe, HH BeHz 180° H-BeH F F-As: ASF5 'F F
> Molecule VSEPR shape Bond angle Polar, non-polar, lon Molecule VSEPR shape Bond angle Polar, non-polar, ion SO32 SO3 SO2 CO2 NO: ВеBrz SCl2 BF3 clo H30
> For this discussion, find a covalently bonded molecule that has a particular shape. Post the following information: Name of the molecule Chemical Formula Empirical Formula Embed an image of the structure Shape of the molecule around the central atom,
> 44. This is the strongest type of covalent bond. a. Ionic b. Hydrogen c. Diatomic d. Dispersion e. Polar 45. This type of solution conducts electricity. a. Electrolyte b. Nonelectrolyte c. Saturated d. Unsaturated e. Supersaturated 46. What is
> For the covalent bonded molecule Oxygen please provide the following information: - Chemical Formula - Empirical Formula - Embed an image of the structure -Shape of the molecule around the central atom, or choose an atom to demonstrate the shape -Use the
> Given that, Calculate the value of ΔH°rxn for H,(e) + F,lg) → 2HF(g) AH=- 546.6 kJ • mol rxn 2H,(2) + 0,le) → 2H,0() AH= - 571.6 kJ· mol :- 571.6 kJ · mol 2F,(g) + 2H,0() → 4HF{g)+0,(g)
> How many σ and π bonds are in this molecule?
> What is the bond angle of dsp³ and what is the bond angle of d²sp³?
> Draw the best Lewis Structure for each of the following structures. Determine the electron and molecular geometries for each using the VSEPR chart. 1. CH4 2. H2O 3. NH3 15. O3
> Record the name, formula and pH of each salt solution. If the pH is not neutral, for the ions that undergo hydrolysis, write down the hydrolysis reaction and indicate which species is responsible for the observed pH. pH of sodium chloride 7.07 pH of a
> Fill in the following chart: Molecular Formula Lewis Structure # of Hybrid- Molecular Electronic |Geometry Geometry Molecular Вond Lewis Structure Electron Formula ization Angle(s) Groups CH.CI: OPCI, (violate the octet rule) CH:O NO: PCIS (violate
> Complete the following chart of electronic and molecular structure. # Electron Domains Molecular Geometry Electron Lewis Diagram # Lone Pairs Geometry H:N:H .. H:C:H H H:0:H
> 11. Using the provided chart (under assignments tab) what is the molecular geometry of NF3 a. Bent b. Tetrahedral c. Trigonal pyramidal d. Trigonal planar 12. Using the provided chart what is the molecular geometry of OCS a. Linear b. Tetrahedral
> Determine the Molecule Geometry (refer to the molecular geometry chart given) and Polarity of the following molecules. It is strongly recommended that you also build the molecules using a molecular modeling kit to aid in your determination of the geometr
> Which statement best describes the polarity of SCL4F2? The molecule is always polar. The molecule is always nonpolar. Depending on the arrangement of outer atoms, this molecule could be polar or nonpolar.
> Name the following compounds: a. Spell out the full name of the compound. b. Spell out the full name of the compound. Br OH Br CH3 HO-
> Describe heterocyclic aromatic compounds.
> Which of the following statement concerning aromatic compounds is CORRECT? A. A linear system of conjugated pi electrons is characteristic of aromatic compounds. B. Aromatic compounds contain only carbon atoms in their pi bonding system. C. Hydrogenat
> Which of the following statements is true? 1. Aromatic compounds release more energy on combustion than aliphatic compounds do. 2. Aromatic compounds react faster than aliphatic compounds in radical halogenation. 3. Aromatic compounds release more ene
> Which of the following statements is true? a. Aromatic compounds have longer C-C bonds than CH2-CH2. b. Aromatic compounds release more energy on combustion than aliphatic compounds do. c. Aromatic compounds release more energy per pi bond upon saturat
> Name the following alkenes using the systematic names. H3C CH2 CH CH H3C CH CH2 ČH3 3,4-methylhex-1-ene CH CH CH2 CH CH2 CH2 ČH3 CH CH2 CH2 H3C
> What is the IUPAC name for the following compound? Enter the name of the molecule. CH, CH,CH,CH, сH, —с —сн, —сH—CH, ČH,CH,
> Which statement is true? a. Aromatic compounds release more energy per pi bond upon saturation than simple alkenes b. Aromatic compounds release more energy on combustion than aliphatic compounds do 5 c. Aromatic compounds react faster than aliphatic c
> Define: aromatic ions anti-aromatic nonaromatic benzenoid aromatic compounds non-benzenoid aromatic compounds
> 3. Anti-aromatic compound doesn't follow the one rule with aromatic compound. Write down that rule? 4. What is the Huckle rule for anti-aromatic compound?
> Which of the following statements is true about aromatic compounds? Select the correct answer below: a. Aromatic compounds undergo substitution reactions more readily than addition reactions. b. Aromatic compounds have properties similar to alkenes.
> Circle the aromatic compounds, put a RECTANGLE around ANTI-AROMATIC Compounds, and for the non-aromatic compounds, explain why they are non-aromatic: HO, -NH2 NH2 HN
> Identify true statements concerning aromaticity. Aromatic compounds must contain a ring. Aromatic compounds must have an even number of pi-electron pairs. Aromatic compounds must have have 4n+2 pi-electrons. Aromatic compounds cannot be ionic.
> Identify the following compounds as non-aromatic, aromatic or anti-aromatic. Non-aromatic compounds Aromatic compounds Anti-aromatic compounds D F 0: 0:
> Give the conjugate acid for each compound below. Base Conjugate Acid H2PPO4- ? HPO42- ? NH3 ?
> Which one of the following compounds is antiaromatic? 14 Which one of the following compound ianmatie II IV A) I B) II C) III D) IV E) none of these 15. Which one of the following compounds is antiaromatic? H V II III IV A) I B) II C) II D) IV E) V
> What mass of ammonium chloride, NH4Cl, (Ka = 5.6 x 10^-10) must be added to exactly 500mL of 0.10M NH3 solution to give a solution with a pH of 9.00?
> Draw the Lewis dot structure and draw the hybridized binding structure (just show bonding orbitals) for each of the following molecules. 1. HCN 2. PH3 3. CH3OCH3 4. COCl2 5. NF6- 6. XeF4
> Lewis Dot (include all resonance structures here; *check Name of formal charge to determine preferred structure, if any) Electron Pair Name of Molecular Bond Bond Dipole Lewis Dot (include all resonance structures here: *check Name of Electron Pair N
> For the following molecules give: a) the Lewis dot structure, and b) a drawing of the 3D structure using VSEPR. 1. PCl5 2. BCl3 3. SiF62- 4. XeF4 5. SO3 6. ICl4 -
> Geometrical Structure of Molecules Lewis Structure Electron geometry Molecular Geometry Polar? 1. CH4 2. CH2Cl2 3. CH3OH 4. H2O 5. Н3O+ 6. HF 7. NH3 8. H2O2 9. N2 10. BrI2 11. C2H4 12. C2H4Br2 13. C2H2 14. SO2 15. SO4-2 16. CO2 17. CSN 18. NO3
> Draw the Lewis structure for the following molecule: OPBr3 Which do not obey the octet rule? NO, BF3, ICl2-, OPBr3, XeF4
> What is the best definition of the reaction quotient, Q? A. The reaction quotient is the ratio of the product of the reactant concentrations when a reaction is at equilibrium. B. The reaction quotient is the product of the reactant concentrations to the
> Compare the reaction quotient to Keq. What does it mean when the reaction quotient is greater than Keq, and vice versa?
> a. Write the reaction quotient for A+2B -> 3C. b. Write the reaction quotient for 2A + B -> 2C+ D
> How is the reaction quotient similar from an equilibrium constant for a reaction? Explain. How is the reaction quotient different from an equilibrium constant for a reaction? Explain.
> Post-Lab Experiment 11 XeF4 Lewis Structure VSEPR Structure of electron groups XeF4 Lewis Structure VSEPR Structure of electron groups electron configuration shape orbital hybridization F-Xe-F bond angle
> What will be the reaction quotient expression for the following equilibrium: 2A +B - C+ 2D
> How many groups are in the periodic table? How many periods are in the periodic table?
> Which of the following pairings is incorrect? Au-d area of periodic table Be-s area of periodic table I-s area of periodic table Xe-p area of periodic table
> The molecular geometry of the IF 5 molecule is ____________. trigonal bipyramidal, nonpolar square pyramidal, nonpolar square pyramidal, polar trigonal bipyramidal, polar trigonal planar, polar
> Determine the electron geometry (eg). molecular geometry (mg) and polarity of TeClsF molecule. (Te is in group 6A, Cl and F are in group 7A) a. eg-octahedral, mg-octahedral, nonpolar b. eg-octahedral, mg-octahedral, polar c. eg-trigonal bipyramidal, m
> Balance the equation and write the reaction-quotient expression, Qc. For the generic reaction The reaction quotient is uls) + 3F,(s) = UF,(s) - UF (e) aAlg) + bB{g) cClg) + dDlg)
> Choose the best Lewis is structure for SeO42- Determine the electron geometry and polarity of SF6. a. eg = trigonal bipyramidal, mg=trigonal bipyramidal, nonpolar b. eg=trigonal bipyramidal, mg=see-saw, polar c. eg=octahedral, mg=trigonal bipyramida
> Draw the Lewis structure of PF, and then determine its electron domain and molecular geometries. A. trigonal bipyramidal / trigonal bipyramidal B. square / octahedral C. planar / trigonal bipyramidal D. tetrahedral / square planar E. tetrahedral / s
> For the following complex, [ML5], the possible coordination geometries are. A. Square-pyramidal and Trigonal-bypyramidal B. Square planar and Trigonal-bipyramidal C. Tetrahedral and Trigonal-bipyramidal D. Octahedral and Trigonal-bipyramidal
> Give the electron-domain and molecular geometries for the following molecules and ions. Part A). HCN electron-domain geometry Linear Trigonal planar Tetrahedral Trigonal bipyramidal Octahedral Part B). HCN molecular geometry Linear Bent Trigonal
> Draw a Lewis dot structure for XeF4.
> Determine the electron geometry (eg) and molecular geometry (mg) of PI5. eg = trigonal planar, mg = octahedral eg = octahedral, mg = square pyramidal eg = tetrahedral, mg = octahedral eg = trigonal bipyramidal, mg = trigonal bipyramidal eg = trigonal
> For ClF3, the electron-pair geometry is _________ and the molecular geometry is __________ 1. trigonal planar, T shape 2. see saw, trigonal bipyramidal 3. trigonal bipyramidal, Tshape 4. tetrahedral, trigonal bipyramidal 5. tetrahedral, tetrahedra
> Determine the electron geometry (eg) and molecular geometry (mg) of ICl2. A. eg = trigonal bipyramidal, mg = linear B. eg = tetrahedral, mg = trigonal pyramidal C. eg = octahedral, mg = bent D. eg = trigonal bipyramidal, mg = tetrahedral E. eg = tetrah
> Determine the molecular shape of each of the following species. (a) CIF3 (b) NF3 (c) CCI4 bent bent bent linear linear linear see-saw see-saw see-saw square planar square planar square planar tetrahedral tetrahedral tetrahedral trigonal bipyramidal
> Determine the electron geometry (eg) and molecular geometry (mg) of PF5. eg=trigonal bipyramidal, mg=trigonal bipyramidal eg=octahedral, mg-octahedral eg=trigonal bipyramidal, mg=tetrahedral eg=tetrahedral, mg=trigonal pyramidal eg=trigonal planar,
> Carbon tetrachloride (CCl4): linear trigonal planar tetrahedral trigonal bipyramidal octahedral Carbon disulfide (CS2): linear trigonal planar tetrahedral trigonal bipyramidal octahedral Ammonia (NH3): linear trigonal planar tetrahedral t
> Write reaction quotients for the following reactions. Do not include states in the reaction quotient. (a) 2NO(g) + 02(g) N204(8) (b) HCOOH(ag) HC00 (aq) + H"(aq)
> 4. AsFs (As has an expanded octet, greater than 8 electrons) a. Trigonal pyramidal AND trigonal pyramidal b. Trigonal pyramidal AND trigonal bipyramidal c. Trigonal bipyramidal AND trigonal pyramidal d. Trigonal bipyramidal AND trigonal bipyramidal
> 1. Identify the electronic geometry and molecular geometry for bromine pentacloride: trigonal bipyramidal, trigonal bipyramidal trigonal bipyramidal, T-shaped trigonal bipyramidal, see saw octahedral, square pyramidal octahedral, octahedral 2. How many
> Determine the electron geometry, molecular geometry and polarity of XeF6. A. eg=octahedral, mg=octahedral, nonpolar B. eg=trigonal bipyramidal, mg=trigonal bipyramidal, nonpolar C. eg=trigonal bipyramidal, mg=see-saw, polar D. eg=octahedral, mg=trigo
> Draw the major product of the following reaction. (3 bookmarks) Draw the major product of the following reaction. KOC(CH,), HOC(CH,), Draw the alky lodide starting material that would give the following alkene as the major product of an E2 reaction
> Draw the electron-dot structure for CHClO. Note: Carbon is the central atom and all three atoms (H, Cl, O) are attached to the carbon. Draw the molecule by placing the atoms on the grid and connecting them with bonds. Include all lone pairs of electrons.
> The electron domain and molecular geometry of SF4 are a. trigonal bipyramidal, seesaw b. trigonal bipyramidal, trigonal bipyramidal c. octahedral, square planar d. octahedral, square pyramidal e. trigonal bipyramidal, T-shaped
> Using the VSEPR model, the electron-domain geometry and the molecular geometry of the central atom in SF6 is A. trigonal bipyramidal; T-shaped B. tetrahedral; tetrahedral C. trigonal bipyramidal; trigonal bipyramidal D. trigonal bipyramidal; seesaw (
> The electron domain geometry, molecular geometry, and polarity of IF5 are _____, _____, and _____, respectively. A. Trigonal bipyramidal, Trigonal bipyramidal, and polar B. Octahedral, trigonal bipyramidal, and non-polar C. Octahedral, trigonal bipyra
> The electron group geometry of AsCl3 is _______ and its molecular geometry is ________. Select one a. trigonal bipyramidal, trigonal bipyramidal b. tetrahedral, trigonal pyramidal c. octahedral; trigonal bipyramidal d. tetrahedral, tetrahedral e tr
> he molecules CH4 and CH3Cl both contains four bonds. Why is CH4 Nonpolar whereas CH3Clis polar?
> The molecular geometry of the CH4 molecule is ________ and the molecule is ________ seesaw, nonpolar linear, polar trigonal pyramidal, polar tetrahedral, polar tetrahedral, nonpolar
> Find the reaction quotient for the reversible reaction below. 2NO(9) + C2(9) = 2NOCI(g)
> What is the molecular geometry of CO2?
> Indicate the electron pair geometry and the molecular geometry for each of the six compounds. Compound Electron palr geometry Molecular geometry CO2 BF3 SO2 SICI4 PF3 OF2
> Indicate the electron pair geometry and the molecular geometry for each of the six compounds. Molecular geometry Compound Electron pair geometry CO, SO, so, CH PF, OF
> Mixture - show any isomers, use multiple bonds if needed, and show any nonzero formal charges. # of val. e's Molecular VSEPR 3-D Structure Formula Lewis Structure Geometry (show polarity) N, co, PBr, scI, CH,0 [(H,C),CO] or CH,COCH,
> Vesper polar Geometry Molecular Lewis Formula Structure Electron Electron Pairs of Geometry Polar Total H of Geometry Lone Molecular Bonds Molecule Total of Geometry Electron Domains Molecule polar Yes Vesper Melecular Formula Lewis Structure Lone Pa
> Indicate the electron pair geometry and the molecular geometry for each of the six compounds. Compound Electron pair geometry Molecular geometry CO, BF, so, CH4 PF3 SCI,
> b. CO2 Electron-Domain Geometry Molecular Geometry: Bond angles: b. CO3 Electron-Domain Geometry: Molecular Geometry: Bond angles:
> Molecules and lons with One Central Atom COâ‚‚ Molecular geometry Ideal bond angles o=c=0 Are polar bonds present? Molecular geometry CO. Ideal bond angles Are polar bonds present? (Indicate on Lewis dot structure.) Is there an overa
> Formula Lewis Dot Structure Drawing Geometry Electron pair geometry Polarity CO2 Molecular geometry CO Electron pair geometry Geometry Electron pair geometry Formula Lewis Dot Structure Drawing Polarity CO2 Molecular geometry Co Electron pair geometr
> Sketch the shape of the following, name the geometry, then draw a dipole arrow if the species is a dipole. AsCls BrFz CBR4 CIO, Molecular Geometry: Molecular Geometry: Molecular Geometry: Molecular Geometry: CO2 CO,- ICL CS2 Molecular Geometry: Molec
> Write an ionic compound from the cation and anion using the correct subscripts and name it. 4. Write an ionic compound from the cation and anion using the correct subscripts and Name it. Cation Formula Name Anion Iron (III) Fe FeCl Iron (III) chlorid
> Arrange each of the following sets of bond in order of increasing polity. Make sure to explain your answers. H-Br, H-F, H-CL, H-I C-H, N-H, F-H, O-H CO, C-C, C-F, C-N spa 8. Complete the below table following the example shown. Counting Electrons & E

