Question: The linear structure of d-glucose is
The linear structure of d-glucose is shown in Figure 16.7. Draw its mirror image.
Figure 16.7:
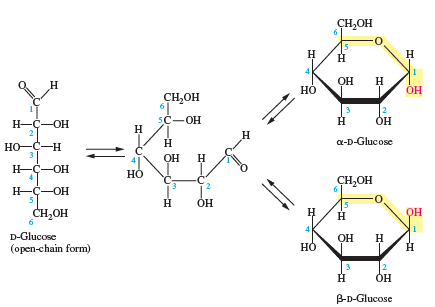
Transcribed Image Text:
CH,OH H H H но OH CH,OH H-C-OH 5C-OH Он H а-D-Glucose HO-C-H 3 OH H H-Ç-OH Но HO'H) -C E. H-C-OH 5 H OH CH,OH он H D-Glucose (орen-chain form) но H 2 ОН В-D-Glucose 2.
> Solutions containing Mg(NO3)2(aq) and NaOH(aq) are mixed. Will a precipitate form? If so, write its formula.
> What do you predict the physical state would be of a triglyceride with three unsaturated fatty acid tails? Explain your reasoning.
> Why are triglycerides also referred to as triacylglycerols?
> Define the term phosphatidate.
> What is the recommendation of the National Institutes of Health for intake of DHA, EPA, linoleic acid, and linolenic acid?
> List some foods that are good sources of a-linolenic acid.
> What foods are good sources of EPA and DHA?
> What are the functions of thromboxane A2 and leukotrienes?
> What is the role of prostaglandins in the inflammatory response?
> What molecules are formed from arachidonic acid?
> Write an equation for the esterification of glycerol with a molecule of capric acid, a molecule of oleic acid, and a molecule of stearic acid.
> a. Define feedback inhibition. b. Describe the role of allosteric enzymes in feedback inhibition. c. Is this positive or negative allosterism?
> Write an equation for the base-catalyzed hydrolysis of a triglyceride containing a molecule of palmitoleic acid, a molecule of oleic acid, and a molecule of palmitic acid.
> Write the complete equation for the esterification of arachidic acid and ethyl alcohol. Write the IUPAC name for each of the organic reactants and products.
> Write equations for the reactions of lauric acid and linoleic acid with KOH.
> Write an equation for the esterification of glycerol with three molecules of palmitic acid.
> Draw the structures of each of the following fatty acids: a. trans-5-Decenoic acid b. cis-5-Decenoic acid
> What is an aldotriose?
> As the number of carbon-carbon double bonds in fatty acids increases, what is the effect on the melting point?
> Write the structures for a saturated and an unsaturated fatty acid.
> Why are lipids (triglycerides) such an efficient molecule for the storage of energy in the body?
> What is the role of lysosomes in the metabolism of plasma lipoproteins?
> Why is resonance an important concept in bonding?
> What is meant by the term fused ring?
> Using condensed formulas, draw the mono-, di-, and triglycerides that would result from the esterification of glycerol with each of the following fatty acids. a. Palmitic acid b. Lauric acid
> Explain why organophosphates are considered to be poisons.
> How do antihistamines function to control the allergic response?
> How does Prozac relieve the symptoms of depression?
> What is the starting material in the synthesis of dopamine, epinephrine, and norepinephrine?
> List some examples of heteropolysaccharides. (Hint: Refer to A Medical Perspective: Monosaccharide Derivatives and Heteropolysaccharides of Medical Interest.)
> What products are formed when methyl o-bromobenzoate reacts with each of the following? a. Aqueous acid and heat b. Aqueous base and heat
> What is the major structural form of sugar in a plant?
> Complete each of the following reactions by supplying the missing product(s). a. CH3NH2 1 HI −−−−→? b. CH3CH2NH2 1 HBr −−−−→? c. (CH3CH2)2NH 1 HCl −−−−→?
> Which is more likely to be a silent mutation, a point mutation or a deletion mutation? Explain your reasoning.
> What form of sugar is used as the major transport sugar in a plant?
> What is the name of the amide bond formed between two amino acids?
> What are the major physiological effects of galactosemia?
> Sucrose is a disaccharide formed by linking a-d-glucose and β-d-fructose by an (a1 → β2) bond. Draw the structure of this disaccharide.
> What is an acetal?
> Explain why ketoses can be oxidized in the Benedict’s test, in contrast to ketones which cannot.
> What is a chiral carbon atom?
> When an acid anhydride and an amine are combined, an amide is formed. This approach may be used to synthesize acetaminophen, the active ingredient in Tylenol. Using the reactants provided here, draw the structure of the amide product, acetaminophen:
> In the Fischer Projections you drew for Practice Problem 16.2 at the end of Example 16.2, indicate which bonds project toward you and which project into the page. Practice Problem 16.2: Draw Fischer Projections for each of the following molecules and f
> Which of the following ionic compounds will form a precipitate in water? a. PbCO3 b. Na2CO3 c. Pb(NO3)2 d. Na2NO3
> Explain why the cyclization of d-glucose forms a hemiacetal.
> Draw all the possible stereoisomers of each of the following compounds, and indicate which are enantiomers, diastereomers, or meso compounds. a. b. CH2OHCHOHCHClCH2OH CH,ČCHOHCHOHČCH,
> Draw all the possible stereoisomers of each of the following compounds, and indicate which are enantiomers, diastereomers, or meso compounds. a. b. ÇOOH H- -H H- -Br H- CI H- н COOH CHO H- OH H- -Br CHO
> Draw a Fischer Projection formula for each of the following compounds. Indicate each of the chiral carbons with an asterisk (*). a. b. c. С-Н H-Ć-H HO-C-H Но-С—Н HO-C-H ČH,OH C-H H-C-H Н-С—ОН он CH,OH С-Н HO-C-H HO-C-H НО-С—Н Н-С—ОН HO-C-H ČH,OH
> Determine whether each of the following is a d- or l-sugar: a. b. c. CH H- -OH Он H -ОН CH,OH CH H- -OH H- FOH HO -H- ČHOH CH H- -O- HO -H ČH,OH
> Erythritol is a four-carbon sugar alcohol and xylitol is a five-carbon sugar alcohol. Like sorbitol and mannitol, they are used as sugar substitutes. Xylitol has the additional benefit of reducing dental cavities and promoting remineralization of the tee
> Define the term meso compound.
> Explain the difference between: a. A ketohexose and an aldohexose b. A triose and a pentose
> Why is the salt of an amine appreciably more soluble in water than the amine from which it was formed?
> Label each of the following statements as true or false and explain why. a. A reaction is at equilibrium when no reactants remain. b. A reaction at equilibrium is undergoing continual change.
> Write an equation for the reaction that would produce each of the following amines: a. Octanamine b. N-Methylpropanamine c. N, N-Diethylpentanamine
> Draw an aldotetrose. Note each chiral carbon with an asterisk (*).
> Define the term enantiomer.
> Draw all of the different possible aldotetroses of molecular formula C4H8O4.
> Draw the open-chain form of the sugars in Question 16.27. Question 16.27: Identify each of the following sugars. CH,OH CH,OH a. C. H. O OH // Но /H/ H. OH H HOOH H. H Но H. ОН H ОН b. НОСН, OH н но, CH-OH H ОН Н
> What is a hexose?
> Define the term ketose.
> Draw and provide the name of an aldotriose.
> Refer to Figure 15.2 and draw hydrogen bonding between two primary amines. Figure 15.2: H H H H H H 'N H 8- 8+ H H 'N H H H H H °C H H H H N H 'N H H H H H H H H 8* H H H (а) (b) do to to E io to
> Some disaccharides are often referred to by their common names. What are the chemical names of (a) milk sugar, (b) beet sugar, and (c) cane sugar?
> How many g of helium must be added to a balloon containing 8.00 g helium gas to double its volume? Assume no change in temperature or pressure.
> What is a polysaccharide?
> What is the function of cellulose in the human diet? How does this relate to the structure of cellulose?
> Show the structure of the thioester that would be formed between coenzyme A and stearic acid.
> Draw the structure of l-galactose.
> Why is L-dopa used to treat Parkinson’s disease rather than dopamine?
> What are the two general classes of neurotransmitters? What distinguishes them from one another?
> Does glycine have a chiral carbon? Explain your reasoning.
> Draw a dipeptide composed of glycine and alanine. Begin by drawing glycine with its amino group on the left. Circle the amide bond.
> Write general equations for the synthesis of a primary, secondary, and tertiary amide.
> Write two equations for the synthesis of each of the following amides. In one equation, use an acid chloride as a reactant. In the second equation, use an acid anhydride. a. Ethanamide b. N-Propylpentanamide c. Propionamide
> Of what significance is it that lysosomal enzymes have a pH optimum of 4.8?
> Complete each of the following by supplying the missing reagents. Draw the structures of each of the reactants and products. a. N-Methylpropanamide +? −−−−→ propanoic acid +? b. N, N-Dimethylacetamide + strong acid −−−−→? + ? c. Formamide 1 strong acid −
> The structure of saccharin, an artificial sweetener, is shown. Circle the amide group. NH is Saccharin
> Locate the amine functional group in the structure of lidocaine. Is lidocaine a primary, secondary, or tertiary amine?
> The amide bond is stabilized by resonance. Draw the contributing resonance forms of the amide bond.
> Draw the condensed formula for each of the following compounds. a. Diethyl methylamine b. 4-Methylpentylamine c. N-Methylaniline d. Triisopropylamine e. Methyl-t-butylamine f. Ethyl hexylamine
> Draw the condensed formula and line formula of each of the following amides: a. Acetamide b. 4-Methylpentanamide c. N, N-Dimethylpropanamide d. Formamide e. N-Ethylpropionamide
> Draw the condensed formula for each of the following amides: a. N-Propylbutanamide b. N-Butyl octanamide c. N-Methyl propanamide d. N-Isopropyl hexanamide
> Use the IUPAC Nomenclature System to name each of the following amides: CH,CH,CHCH,CNH, CH,CHČNH, 'H? CH, с. Br b. -ČNH, Br
> Why is acetaminophen often recommended in place of aspirin?
> How are the common names of amides derived?
> Will the rate of the reaction in Question 7.61 increase, decrease, or remain the same if the concentration of methane increases? Question 7.61: CH4 (g) + 2O2 (g)−−→2H2O(l) + CO2 (g)
> How do the names of the first three enzymes of the glycolytic pathway relate to the reactions they catalyze?
> Describe the water solubility of amides in relation to their carbon chain length.
> Distinguish between the term’s analgesic and anesthetic.
> What is an alkaloid?
> How would you quickly convert an alkylammonium salt into a water-insoluble amine? Explain the rationale for your answer.
> Why does aspirin upset the stomach, whereas acetaminophen (Tylenol) does not?
> Name each of the following amines using the systematic and common nomenclature systems. CH3 NH2 NH а. CH-CHCHCH3 c. CH3CHCH,CH3 NH2 CH3 N-CH,CH3 d. CH;CHCH3 b. CH-C-CH3 ČH3
> Complete each of the following equations by supplying the missing reactant or product indicated by a question mark: a. b. c. d. CH,CH,NH2 + H;O ? + OH- CH,CH,CH, ? + HCI CH;CH;CH,N+HC- CH, CH,CHNH + HạO - ? + ? NH3 + HBr ?
> Name each of the following carboxylic acids, using both the common and IUPAC Nomenclature Systems: Br a. CH,CH,CHCHCH,C-OH CH, с. С-ОН -OH CH,CH, O b. CH,CH,CHCH,CH,C-OH CH2
> Classify each of the following amines as primary, secondary, or tertiary: a. Benzenamine b. N-Ethyl-2-pentanamine c. Ethyl methylamine d. Tripropylamine e. m-Chloroaniline
> Draw all of the isomeric amines of molecular formula C3H9N. Name each of the isomers using the systematic names, and determine whether each isomer is a primary, secondary, or tertiary amine.
> What is the H3O+ concentration of a solution with a pH of: a. 6.80 b. 4.60 c. 2.70
> Draw the condensed formula for each of the following compounds: a. 2,3-Dibromoaniline b. 2-Octanamine c. 2-Chloro-2-pentanamine d. N, N-Diethyl pentanamine e. Diisopropylamine

