Question: We will see in Chapter 20 that
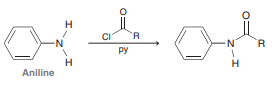
We will see in Chapter 20 that derivatives of aniline will react with acyl chlorides (RCOCl) in the presence of pyridine (a base) to yield aromatic amides, as shown below for the parent aniline:
Transcribed Image Text:
'R -N- N' 'R PY Aniline
> Compound A has the molecular formula C7H14O and reacts with sodium borohydride in methanol to form an alcohol. The 1H NMR spectrum of compound A exhibits only two signals: a doublet (I = 12) and a septet (I = 2). Treating compound A with 1,2-ethanedithio
> Historically, the nitroso group has been known as a strong deactivator, yet an ortho/para-director, in electrophilic aromatic substitution reactions. For example, nitration of nitrosobenzene affords mostly the para-substituted product. a. Rationalize th
> When ortho-bromonitrobenzene is treated with NaOH at elevated temperature, only one product is formed. a. Draw the product. b. Identify the intermediate formed en route to the product. c. Would the reaction occur if the starting compound were metabromo
> Predict the major product of the following reaction: HO,S. So,H ? Dilute HSO.
> When the following compound is treated with Br2 in the presence of a Lewis acid, one product predominates. Determine the structure of that product. Br2 FeBra ?
> The following compound is highly activated but nevertheless undergoes bromination very slowly. Explain.
> Identify the major product formed when each of the following compounds is treated with Et2CuLi followed by mild acid: CN OEt (a) (b) (c)
> When benzene is treated with methyl chloride and aluminum trichloride under conditions that favor trialkylation, one major product is obtained. Draw this product and provide an IUPAC name.
> Starting with benzene and using any other reagents of your choice, design a synthesis for each of the following compounds. In some cases, there may be more than one plausible answer. OMe COOH Br- Br NO2 Rr (a) NO2 (b) COOH (c) NO2 OEt COOH NO, .CI NO
> Each of the following compounds can be made with a Friedel– Crafts acylation. Identify the acyl chloride and the aromatic compound you would use to produce each compound. O,N. (a) OCH, LOCH, H,CO (b)
> When 2,4-dibromo-3-methyltoluene is treated with bromine in the presence of iron (Fe), a compound with the molecular formula C8H7Br3 is obtained. Identify the structure of this product.
> Predict the product(s) for each of the following reactions: Br ? AICI, (a) HNO, H,SO, OCH, (b) ? Fuming H,SO, (c) HNO, H2SO4 ? (d) Br NO2 오
> Propose an efficient synthesis for each of the following transformations: OCH, OCH, OCH, OCH, Br NO, NO2 Br (b) (a) HO NO, `NO2 (c) (d)
> Compound A and compound B are both aromatic esters with the molecular formula C8H8O2. When treated with bromine in the presence of iron tribromide, compound A is converted into only one product, while compound B is converted into two different monobromi
> For each of the following groups of compounds, identify which compound will react most rapidly with ethyl chloride in the presence of aluminum trichloride. Explain your choice in each case and then predict the expected products of that reaction. CN C
> Draw all resonance structures of the sigma complex formed when toluene undergoes chlorination at the para position.
> Benzene was treated with isopropyl chloride in the presence of aluminum trichloride under conditions that favor dialkylation. Draw the major product expected from this reaction.
> Compound 2, a pheromone of the honeybee queen, has been shown to have antibacterial and anti-inflammatory properties. One laboratory synthesis of this pheromone involved the preparation of 1, followed by the conversion of 1 into 2. Using an acetoacetic e
> Propose a plausible mechanism forthe following transformation: он OH
> Picric acid is a military explosive formed via the nitration of phenol under conditions that install three nitro groups. Draw the structure and provide an IUPAC name for picric acid.
> When para-bromotoluene is treated with sodium amide, two products are obtained. Draw both products and propose a plausible mechanism for their formation.
> In each case, identify the most likely position at which monobromination would occur. (a) (b) (c)
> Each of the following syntheses will not produce the desired product. In each case, identify the flaw in the synthesis. NO 1) HNO,/H,SO, 2) EICI, AICI, (a) Et Br (b)|| 1) Brg. FeBra 2) AICI, Br 1) Brg. FeBra (c) 2) AICI, Br 1) AICI, 2) Br2, FeBra (d
> Starting with benzene and using any other reagents of your choice, design a synthesis for each of the following compounds: H,N Br H,N. (a) (b)
> Predict the product(s) of the following reactions: 1) HNO,/H,SO, ? 2) Zn, HCI 3) NaOH (a) Br 1) AICI CI 2) Zn(Hg), HCI, heat ? (b) 1) CH;CI, AICI, 2) KMnO, Hő, heat ? 3) H,0" (c) 1) CH;CI, AICI3 2) Excess NBS ? (d)
> Propose a plausible mechanism for the following transformation: NaOMe + Naci OMe
> Propose a plausible mechanism for each of the following transformations: I-CI AICI (a) CH,Cl, AICI, (b)
> Draw a mechanism for each of the following transformations: NO, HNO, (a) (b) so,H Fuming CH,GI H,SO, AICI, (c) (d) Br Br2 Fe (e)
> Propose an efficient synthesis for each of the following compounds using an acetoacetic ester synthesis: (a) (Ь) (d)
> When benzene is treated with 2-methylpropene and sulfuric acid, the product obtained is tert-butylbenzene. Propose a mechanism for this transformation.
> When the following compound is treated with a mixture of nitric acid and sulfuric acid at 50°C, nitration occurs to afford a compound with two nitro groups. Draw the structure of this product: ? HNO/H2SO4
> Predict the major product obtained when each of the following compounds is treated with bromine in the presence of iron tribromide: a. Bromobenzene b. Nitrobenzene c. ortho-Xylene d. tert-Butylbenzene e. Benzenesulfonic acid f. Benzoic acid g. Ben
> Predict the product(s) obtained when each of the following compounds is treated with chloromethane and aluminum trichloride. Some of the compounds might be unreactive. For those that are reactive, assume that conditions are controlled to favor monoalkyla
> The following compound has four aromatic rings. Rank them in terms of increasing reactivity toward electrophilic aromatic substitution. -N
> For each of the following groups, identify whether it is an activator or a deactivator, and determine its directing effects: (a)FOMo (b) -o (c)-NH, ci (d) -CCI, (F) {-NO, HO. H. (g) (h) (1) -Br -NMe,
> Predict the major product obtained when each of the following compounds is treated with fuming sulfuric acid: a. Chlorobenzene b. Phenol c. Benzaldehyde d. ortho-Nitrophenol e. para-Bromotoluene f. Benzoic acid g. para-Ethyltoluene
> Predict the product(s) obtained when each of the following compounds is treated with a mixture of nitric acid and sulfuric acid: Br NO2 (a) (h) (c) OMe (d) (e)
> Identify which of the following compounds is most activated toward electrophilic aromatic substitution. Which compound is least activated? NO2 .Br но, OMe TON
> Rank the following compounds in order of increasing reactivity toward electrophilic aromatic substitution: Br Br. Br
> In a study to prepare new and more effective anti-inflammatory agents, 2-fluoroloxoprofen was prepared from compound 2, which in turn was the product of a malonic ester synthesis. Starting with diethyl malonate, identify reagents that can be used to prep
> Identify reagents that can be used to accomplish each of the following transformations: Br SO,H ON NH, CBr3 HO.
> The welwitindolinones are a class of natural products that exhibit a host of biological activities including insecticidal, fungicidal, and anti-cancer properties. The following reaction was performed using SnCl4, a Lewis acid, in a model study in route t
> When 2-ethyl-5-chlorotoluene was treated with sodium hydroxide at high temperature, followed by treatment with H3O+, three constitutional isomers with the molecular formula C9H12O were obtained. Draw all three products.
> Draw the most likely mechanism for each of the following transformations: Br он Br 1) NaOH, 350°C 2) H30* Br, FeBrg (a) (Ь) O,N. O2N 1) NaNH, 2) H,0 (c) `NH2
> Starting with benzene and using any other necessary reagents of your choice, design a synthesis for anisole (methoxybenzene).
> Draw both products that are obtained when 4-chloro2-methyltoluene is treated with sodium amide followed by treatment with H3O+.
> The presence of additional nitro groups can have an impact on the temperature at which a nucleophilic aromatic substitution will readily occur. Consider the following example: When both R groups are hydrogen atoms, the reaction readily occurs at 130&Aci
> Starting with benzene and using any other necessary reagents of your choice, design a synthesis for the following compound: но- -NH2
> Predict the product of the following reaction: Br, NaOCH,, heat CI- -NO2
> Propose an efficient synthesis for each of the following compounds using the malonic ester synthesis:
> Draw both resonance structures of the enolate formed when each of the following ketones is treated with a strong base: H (e) (Ь) (d)
> Starting with benzene and using any other necessary reagents of your choice, design a synthesis for each of the following compounds. In some cases, there may be more than one plausible answer. Br NH2 Br H,N. Br (a) (Ь) (c) (d)
> 2-Nitroaniline has been used as a precursor in the synthesis of the benzimidazole ring system (shown below), a structural feature that is present in many pharmaceuticals. A reliable method for synthesizing 2-nitroaniline relies on a reaction we will lear
> Starting with benzene and using any other necessary reagents of your choice, design a synthesis for each of the following compounds. Note: some of these problems have more than one plausible answer. NH2 Br O2N H2N (a) (Ь) (c) (d) CI Br Br CBr3 (e) (f
> Identify the product obtained when benzene is treated with each of the following reagents: a. Fuming sulfuric acid b. HNO3 /H2SO4 c. Cl2, AlCl3 d. Ethyl chloride, AlCl3 e. Br2, Fe f. HNO3 /H2SO4 followed by Zn, HCl, followed by NaOH
> Identify reagents that can be used to convert benzene into each of the following compounds: a. Chlorobenzene b. Nitrobenzene c. Bromobenzene d. Ethylbenzene e. Propylbenzene f. Isopropylbenzene g. Aniline (aminobenzene) h. Benzoic acid i. Toluen
> The flavor of beer can be tainted by a trace contaminant, called ortho-bromophenol. To reduce the incidents of contamination, beer manufacturers have used certified beer flavor standards to train professional beer tasters to recognize the flavor of ortho
> Determine whether a blocking group is necessary to accomplish each of the following transformations: (a) NO2 (b) Br Br но. но NO2 (c) (d)
> Sildenafil citrate was the first approved drug for treating male erectile dysfunction, a condition affecting more than 30 million men in the U.S. The following reaction is part of a synthesis of sildenafil, better known as Viagra. Here, an electrophilic
> For each of the following compounds, determine the position that is most likely to be the site of an electrophilic aromatic substitution reaction: O2N OMe HO. NO2 (a) (b) Br OH H. TH. но. (c) (d)
> 4-Fluoro-3-nitroaniline is a patented synthetic precursor used in the production of commercial hair dyes. Identify the position(s) on this compound most likely to undergo electrophilic aromatic substitution. NH2 *NO2
> Identify reagents that can be used to achieve the following transformation: он он
> For each compound below, identify which position(s) is/are most likely to undergo an electrophilic aromatic substitution reaction. CH CH O2N NO2 (a) (b) O,N NO2 (c) CN Br OH (d) (e) (f) OMe Br HO (g) (h) Br (i) O
> Diazepam is a prescription medication first marketed under the trade name Vallium, and used to treat anxiety disorders. Predict whether the monosubstituted aromatic ring in diazepam is activated or deactivated toward electrophilic aromatic substitution,
> For each of the following compounds, predict whether the ring is activated or deactivated, then predict the strength of activation/deactivation, and finally predict the expected directing effects. Br -NO2 (a) (b) (c) N- (d) (e) (f)
> Predict and explain the regiochemical outcome for chlorination of bromobenzene.
> Does chlorination of chlorobenzene require the use of a Lewis acid? Explain why or why not.
> When 1,3-dinitrobenzene is treated with nitric acid and sulfuric acid at elevated temperature, the product is 1,3,5-trinitrobenzene. Explain the regiochemical outcome of this reaction. In other words, explain why nitration takes place at the C5 position.
> When ethoxybenzene is treated with a mixture of nitric acid and sulfuric acid, two products are obtained, each of which has the molecular formula C8H9NO3. a. Draw the structure of each product. b. Propose a mechanism of formation for the major product.
> Draw the two major products obtained when toluene undergoes monobromination.
> A Friedel–Crafts acylation is an electrophilic aromatic substitution in which the electrophile (E+) is an acylium ion. There are other methods of forming acylium ions, such as treatment of an anhydride (shown below) with a Lewis acid. T
> The following compound cannot be made with either a Friedel–Crafts alkylation or acylation. Explain.
> For each of the following reactions, predict the major product and propose a mechanism for its formation: 1) NaH 1) LDA ? ? 1) LDA, -78'C 2) Etl ? 2) CH,I 2) CH,Br 1) LDA, -78'C (a) (b) (c) 2) CH,I
> Identify whether each of the following compounds can be made using a direct Friedel–Crafts alkylation or whether it is necessary to perform an acylation followed by a Clemmensen reduction to avoid carbocation rearrangements: (a) (b)
> A Friedel–Crafts alkylation is an electrophilic aromatic substitution in which the electrophile (E+) is a carbocation. In previous chapters, we have seen other methods of forming carbocations, such as protonation of an alkene using a st
> Draw a mechanism for the following reaction, which involves two consecutive Friedel–Crafts alkylations. AICI3 to
> Predict the expected product(s) when benzene is treated with each of the following alkyl halides in the presence of AlCl3. In each case, assume conditions have been controlled to favor monoalkylation. 'CI (a) (b) (c)
> Draw a mechanism for the following reaction and make sure to draw all three resonance structures of the sigma complex: NO2 HNO, H,SO,
> When benzene is treated with D2SO4, a deuterium atom replaces one of the hydrogen atoms. Propose a mechanism for this reaction. Once again, make sure that your mechanism involves a sigma complex. D;SO,
> Draw a mechanism for the following reaction. Hint: This reaction is the reverse of sulfonation, so you should read the sulfonation mechanism backward. Your mechanism should involve a sigma complex (positively charged). so,H H. Dilute H,SO,
> When benzene is treated with 2 in the presence of CuCl2, iodination of the ring is achieved with modest yields. It is believed that CuCl2 interacts with 2 to generate +, which is an excellent electrophile. The aromatic ring then reacts with + in an elect
> Predict the products for each of the following reactions: CN H. NC CN a) NC (c) Meo COOH CN HOOC d) Meo (f)
> The following synthesis was developed in an effort to prepare an analogue of a polycyclic aromatic hydrocarbon in which one of the C=C bonds was replaced with a B−N unit. Such derivatives are expected to display unique optical and elect
> When the following compound is treated with sodium ethoxide, followed by acid work-up, two condensation products are obtained, both of which are produced via Dieckmann cyclizations. Draw both products. EtO OEt
> Treatment of compound 1 with benzene in triflic acid (CF3SO3H) affords ammonium ion 3. Triflic acid is an extremely strong acid (pKa = −14), even more acidic than sulfuric acid; under these conditions, the transformation is believed to
> Electrophilic addition of HI proceeds with the same mechanism as HCl and HBr. New methods for the hydroiodination of dienes were studied and the reactions consistently produced the thermodynamic addition product. Predict the products of the given reactio
> When 1,4-dimethylcyclohepta-1,3-diene is treated with HBr at elevated temperature, the 1, 2-adduct predominates, rather than the 1,4-adduct. Explain this result. HBr 40°C Br
> Predict the products for each of the following reactions, and in each case, determine which product will predominate: HCI ? ? HBr HBr O°C 40°C O'C (b) (c)
> Consider the following reaction, in which the product results from substitution of fluorine and not from substitution of chlorine. a. Draw a mechanism for this reaction. b. Based on the observed regiochemical outcome, identify the step of the mechanism
> Compound 1 and compound 2 both contain tritium (T), which is an isotope of hydrogen (tritium = 3 H). Both compounds are stable upon treatment with aqueous base. However, upon prolonged treatment with aqueous acid, compounds 1 and 2 both lose tritium, to
> The rate constants for the bromination of several disubstituted stilbenes are given in the table below. Given that the double bond of stilbene acts as the nucleophile, provide a reasonable explanation for the trend observed among the rate constants.
> Aromatic heterocycles are also capable of undergoing electrophilic aromatic substitution. For example, when furan is treated with an electrophile, an electrophilic aromatic substitution reaction occurs in which the electrophile is installed exclusively a
> When N,N-dimethylaniline is treated with bromine, ortho and para products are observed. Yet, when N,N-dimethylaniline is treated with a mixture of nitric acid and sulfuric acid, only the meta product is observed. Explain these curious results. Br Brg
> Propose a plausible mechanism for the following transformation: но. но. H,SO,
> Predict the product of the Dieckmann cyclization that occurs when each of the following compounds is treated with sodium ethoxide, followed by acid work-up: EtO OEt OE! OEt (a) (b) EtO (c) OEt
> Bakelite is one of the first known synthetic polymers and was used to make radio and telephone casings as well as automobile parts in the early twentieth century. Bakelite is formed by treating phenol with formaldehyde under acidic conditions. Draw a pla
> Predict the major product of the following reaction: ? AICI,
> Each of the following compounds is an aromatic compound bearing a substituent that we did not discuss in this chapter. Using the principles that we discussed in this chapter, predict the major product for each of the following reactions: ? HNO, H,SO,
> When toluene is treated with a mixture of excess sulfuric acid and nitric acid at high temperature, a compound is obtained that exhibits only two signals in its 1H NMR spectrum. One signal appears upfield and has an integration of 3. The other signal ap
> The 1 H NMR spectrum of phenol exhibits three signals in the aromatic region of the spectrum. These signals appear at 6.7, 6.8, and 7.2 ppm. Use your understanding of shielding and deshielding effects (Chapter 15) to determine which signal corresponds wi

