Question: Amino acids are biological compounds with the
Amino acids are biological compounds with the following structure, where the R group can vary. The structure and biological function of amino acids will be discussed in Chapter 25. Identify the total number of lone pairs present in an amino acid, assuming that the R group does not contain any atoms with lone pairs.
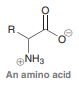
Transcribed Image Text:
R. @NH3 An amino acid
> In the following compound, two protons are clearly identified. Determine which of the two is more acidic. After comparing the conjugate bases, you should get stuck on the following question: Is it more stabilizing for a negative charge to be spread out o
> Ascorbic acid (vitamin C) does not contain a traditional carboxylic acid group, but it is, nevertheless, still fairly acidic (pKa = 4.2). Identify the acidic proton and explain your choice using resonance structures, if necessary: он но HO он Ascorbi
> In each compound below, two protons are color-coded (red and blue). Determine which of the two protons is more acidic: H -N -N- H -H (а) H (Ь) H (с) H. (d) H (e) H (f) H
> Arsenic is an element that can cause a variety of cancers. The Environmental Protection Agency (EPA) and the World Health Organization (WHO) have thus set limits on acceptable levels of arsenic in drinking water. Monitoring these limits in developing cou
> Predict the major and minor products for each of the following E2 reactions: Br ? ? NaOEt NaOEt CI (a) (b) Br ? ? NaOE! NaOE! (c) (d) Br Br ? ? -BUOK -BUOK (e) (f)
> In each compound below, two protons are color-coded (red and blue). Determine which of the two protons is more acidic: H H-Ç-N, N-H HS, H (а) H (Ь) H (с) он (d) н
> Amino acids, such as glycine, are the key building blocks of proteins and will be discussed in greater detail in Chapter 25. At the pH of the stomach, glycine exists predominantly in a protonated form in which there are two acidic protons of interest. Th
> Polyether ether ketone (PEEK) is a biocompatible polymer material that is used to make medical implants. The conjugate base of hydroquinone is used in the synthesis of PEEK. Is hydroxide a strong enough base for deprotonating hydroquinone? to Polyet
> Determine the position of equilibrium for each acid-base reaction below: OH = (a) OH (b) 1 CD I-O H (c) H H. HA H (d) H-cEc-H NH2 H-CEc: NH, 1
> Nicotine is an addictive natural product found in tobacco. It has been proposed that the amount of nicotine taken in by the body may depend on the relative proportions of the free base 1, as compared to its mono- and di-protonated forms, 2 and 3. Using t
> Compound 1 was isolated from a species of Streptomyces bacteria and has been shown to have antibacterial and anticonvulsant properties. Compound 1 has also been made in the laboratory; however, it was prepared as its conjugate acid rather than in its neu
> Identify the stronger base in each of the following cases: (a) H-CEc TH (b) (с) H H H-C-C-H H-CEC e (d) OH (е) н (f) a OH
> L-dopa is used in the treatment of Parkinson’s disease. Using Table 3.1, identify the four most acidic protons in the compound and then arrange them in order of increasing acidity (two of the protons will be very similar in acidity and
> Propranolol is an antihypertensive agent (used to treat high blood pressure). Using Table 3.1, identify the most acidic proton in the compound and indicate the approximate expected pKa. From Table 3.1: OH H Propranolol TABLE 3.1 pK VALUES OF COMMON
> For each pair of compounds below, identify the more acidic compound: H TH (а) (b) O-4 H TH H-CECH (с) н (d) н" C-H H H-C-C-H H-CEC-H H-0-S-o-H (е) н H (f)
> Predict which of the following two compounds will undergo an E2 reaction more rapidly: CI CI
> Dextromethorphan is a commonly used cough suppressant (antitussive) that is under investigation for other therapeutic uses, including treatment of bipolar disorder.1 To increase its shelf-life, the drug is prepared as its hydrobromide salt by treating it
> In an intramolecular proton transfer reaction, the acidic site and the basic site are tethered together in the same structure, and a proton is passed from the acidic region of the structure to the basic region of the structure. An example is shown. Draw
> All of the following acid-base reactions are reactions that we will study in greater detail in the chapters to follow. For each one, draw a mechanism and then clearly label the acid, base, conjugate acid, and conjugate base: он (а) (Ь) H (с) N. O-4 о
> Draw all constitutional isomers with the following molecular formula. a. C3H7Cl b. C4H10 c. C5H12 d. C4H10O e. C3H6Cl2
> Coumarin and its derivatives exhibit a broad array of industrial applications, including, but not limited to, cosmetics, food preservatives, and fluorescent laser dyes. Some derivatives of coumarin, such as warfarin, exhibit antithrombic activity and are
> Polymers are very large compounds, assembled from smaller units, called monomers. For example, polymer 2, called poly(vinyl acetate), is made from vinyl acetate (1). Polymer 2 can be converted to polymer 4, called poly(vinyl alcohol), or PVA, via a proce
> Many compounds with desirable medicinal properties are isolated from natural sources and are thus referred to as natural products. However, a compound’s medicinal properties are often known before the structure of the compound has been
> Progesterone is a female hormone that plays a critical role in the menstrual cycle by preparing the lining of the uterus for implantation of an egg. During W. S. Johnson’s biomimetic synthesis of progesterone (a synthesis that draws ins
> CL-20 and HMX are both powerful explosives. CL-20 produces a more powerful blast but is generally considered too shock-sensitive for practical use. HMX is significantly less sensitive and is used as a standard military explosive. When a 2:1 mixture of th
> Compound 2 is produced by the fungus Exserohilum rostratum and has been shown to fight inflammation and block/prevent tissue scarring. Compound 2 was made in the laboratory from compound 1, as shown. One of the early steps in this process involves the fo
> When menthyl chloride is treated with a strong base, only one elimination product is observed. Yet, when neomenthyl chloride is treated with a strong base, two elimination products are observed. Draw the products and explain. "ci 'CI Neomenthyl chlor
> Ramelteon is a hypnotic agent used in the treatment of insomnia: a. What is the molecular formula of this compound? b. How many sp3 -hybridized carbon atoms are present in this structure? c. How many sp2 -hybridized carbon atoms are presen
> Cycloserineis anantibiotic isolated from the microbe Streptomyces orchidaceous. It is used in conjunction with other drugs for the treatment of tuberculosis. a. What is the molecular formula of this compound? b. How many sp3 -hybridized carbon atoms ar
> Enamines, compounds with an amino group attached to a double bond, are electron-rich alkenes that have important synthetic applications. Draw the major resonance contributors for the enamine shown and determine which site(s) on the structure are most ele
> Draw and rank all significant resonance forms for the following compound. Briefly explain your rankings. HC
> Use resonance structures to help you identify all sites of high electron density (δ−) in the following compound: но OH
> Use resonance structures to help you identify all sites of low electron density (δ+) in the following compound:
> Draw all significant resonance structures for each of the following compounds: OH OH H. HO Estradiol Testosterone (Female sex hormone) (Male sex hormone)
> Melatonin is an animal hormone believed to have a role in regulating the sleep cycle: The structure of melatonin incorporates two nitrogen atoms. What is the hybridization state and geometry of each nitrogen atom? Explain your answer. OCH, H Melaton
> Consider the structure of ozone: Ozone is formed in the upper atmosphere, where it absorbs short wavelength UV radiation emitted by the sun, thereby protecting us from harmful radiation. Draw all significant resonance structures for ozone. (Hint: Begin
> A mixture of sulfuric acid and nitric acid will produce small quantities of the nitronium ion (NO2+): Does the nitronium ion have any significant resonance structures? Why or why not? 0=N=0:
> Retinol, one of the forms of vitamin A, is found in animal food sources and is essential for good vision. Below are two organohalides that were used to make synthetic forms of vitamin A. Upon treatment with a strong base, each of these compounds undergoe
> Draw a bond-line structure for each of the following compounds: a. CH2=CHCH2C(CH3)3 b. (CH3CH2)2CHCH2CH2OH c. CH≡COCH2CH(CH3)2 d. CH3CH2OCH2CH2OCH2CH3 e. (CH3CH2)3CBr f. (CH3)2C=CHCH3
> Consider each pair of compounds below and determine whether the pair represents the same compound, constitutional isomers, or different compounds that are not isomeric at all: (b) (c) (d)
> Determine the relationship between the two structures below. Are they resonance structures or are they constitutional isomers?
> Draw resonance structures for each of the following: (a) (b) (c) 'N NH2 (e) (f) (g) (h) HO. (j)
> Draw bond-line structures for all constitutional isomers with the molecular formula C4H9Cl.
> Identify the number of carbon atoms and hydrogen atoms in the compound below:
> What is the molecular formula for each compound in the previous problem?
> Write a condensed structural formula for each of the following compounds: OH (a) (b) (с)
> Draw all significant resonance structures for each of the following: OH (а) (b) (с)
> Each structure below exhibits one lone pair. In each case, identify the type of atomic orbital that the lone pair occupies. (a) (b) (c)
> Identify the major and minor products for the E2 reaction that occurs when each of the following substrates is treated with a strong base: Br Br Br (а) (Ь) (c) (d) (e) (f) ** (g) (h)
> Identify the number of sp3 -hybridized carbon atoms in the following compound: (CH3)2C=CHC(CH3)3
> Identify which two compounds below are constitutional isomers: (CHa),CÖCH, (CH3)»CHÖCH, (CH,),CHÖCH,CH,
> Draw bond-line structures for all constitutional isomers of the following compound: CH3CH2CH(CH3)2
> Draw significant resonance structures for the following compound:
> Draw the missing formal charge in each case below:
> A carbene is a highly reactive intermediate in which a carbon atom bears a lone pair and no formal charge: How many hydrogen atoms are attached to the central carbon atom above?
> How many lone pairs are found in the structure of vitamin C?
> Draw bond-line structures for vitamin A and vitamin C: H H C-H H H H.H нн HH C. H "C- H HH H Vitamin A H HO, HO но. нн C=C OH но Vitamin C HIC
> Draw bond-line structures for all constitutional isomers of C5H12.
> Adamantane, a tricyclic alkane consisting of fused cyclohexane rings, has an intriguing structure that was first proposed as a theoretical structure in 1924. Chemists set out to synthesize the molecule and succeeded in 1941. Because its structure resembl
> The following substitution reaction exhibits second-order kinetics, and is therefore presumed to occur via an SN2 process: a. What happens to the rate if the concentration of 1-iodopropane is tripled and the concentration of sodium hydroxide remains the
> Draw bond-line structures for all constitutional isomers of C4H10.
> Draw all carbon atoms, hydrogen atoms, and lone pairs for the following compounds: он N. HO Acetylsalicylic acid (aspirin) Acetaminophen (Tylenol) Caffeine
> Isoniazid is used in the treatment of tuberculosis and multiple sclerosis. Identify each lone pair as either localized or delocalized. Justify your answer in each case. NH2 N H Isoniazid
> Nicotine is a toxic substance present in tobacco leaves. There are two lone pairs in the structure of nicotine. In general, localized lone pairs are much more reactive than delocalized lone pairs. With this information in mind, do you expect both lone pa
> For each compound below, identify all lone pairs and indicate whether each lone pair is localized or delocalized. Then, use that information to predict the geometry for each atom that exhibits a lone pair. H2N. NH2 N. (a) (b) 'N' (c) H
> The dragmacidin class of natural products has been isolated from various marine sponges. They have been shown to have many interesting biological properties, including anti-viral, anti-fungal, and anti-bacterial activity. (+)-Dragmacidin D, shown below,
> Draw a resonance hybrid for each of the following. (a) (b) (d) (e) la (g) (h)
> Valderramenol A, a natural product that was isolated from the leaves of a plant native to the Philippines, was found to be antitubercular (can be used to treat tuberculosis). The structure of valderramenol A contains two benzene rings. Using resonance, d
> In the compounds shown below, the six-membered rings are called benzene rings. Such rings are commonly found in natural products, and we will learn more about the remarkable stability of these ring systems in Chapter 17. Because of resonance effects with
> For each of the following, draw all significant resonance forms and rank them from most significant to least significant. Briefly explain the rankings. NH (a) (b) (c) 'N (d) CH3-C-CEN (e) (f)
> For each of the following reactions, identify whether you would use hydroxide or tertbutoxide to accomplish the desired transformation: (Ь) Br (а)
> Draw resonance structures for each of the following compounds: o (a) (b) (f) (h)
> Fingolimod is a novel drug that has been developed for the treatment of multiple sclerosis. In 2008, researchers reported the results of phase III clinical trials of fingolimod, in which 70% of patients who took the drug daily for three years were relaps
> Draw a resonance structure of the compound shown below, called 2-heptanone, which is found in some kinds of cheese.
> Draw a resonance structure of the following compound, which was isolated from the fruits of Ocotea corymbosa, a native plant of the Brazilian Cerrado. он
> Draw a resonance structure for each of the compounds below. (a) (b) (c)
> For each of the compounds below, locate the lone pair adjacent to a positive charge and draw the resonance structure: (a) (b) (c)
> Draw the resonance structure(s) for each of the compounds below: (a) (b) (c) (d)
> For each of the compounds below, locate the pattern we just learned (lone pair next to a π bond) and draw the appropriate resonance structure: NH2 (a) (b) (c) (d) (e) Acetylcholine (g) (a neurotransmitter) 5-Amino-4-oxopentanoic acid (used
> The cation 1 has been shown to lose a proton (H+) to produce a compound represented by resonance structures 2a, 2b, and 2c. a. Draw the curved arrows needed to convert resonance structure 2a into resonance structure 2b. Begin by drawing all lone pairs a
> In each case below, draw the curved arrow(s) required in order to convert the first resonance structure into the second resonance structure. In each case, begin by drawing all lone pairs and then use the formal charges to guide you. (a) (Б) --- (c) (
> Predict the major and minor products for each of the following E2 reactions: -BUOK ? NaOE (a) (b) ? ? NaOH I-BUOK (c) (d) Br Br ? NaOH -BUOK (e) (f)
> For each of the structures below, draw the resonance structure that is indicated by the curved arrows. Be sure to include formal charges. (a) (b) (c) (d) (e) (f) (g) (h)
> Polyhydroxybutyric acid (PHB) is a component of biodegradable plastics that can be used in dissolving sutures or as a scaffold for skin regeneration. PHB is synthesized by linking together hydroxybutyric acid molecules. Inspect the arrows drawn for possi
> Drawing the resonance structure of the following compound requires one curved arrow. The head of this curved arrow is placed on the oxygen atom, and the tail of the curved arrow can only be placed in one location without violating the rules for drawing c
> In each of the following cases, determine whether the curved arrow violates either of the two rules and describe the violation, if any. (Don’t forget to count all hydrogen atoms and all lone pairs.) :ÖH (a) H (ь) (c) (d) (e) (f) (g)
> Glue adhesion usually requires a dry surface, so studying the chemistry behind the attachment of mussels to wet rocks can lead to the development of new adhesive materials. Chemists used the compound shown to model the mussel foot proteins involved in th
> Draw all lone pairs on each of the nitrogen atoms in the following structures. First, review Table 2.3 and then come back to these problems. Try to identify all lone pairs without having to count. Then, count to see if you were correct. From Table 2.3:
> The rich and varied flavors of toasted bread, roasted coffee, and seared meat are a result of a process known as the Maillard reaction. This reaction creates hundreds of new flavorful compounds, including hydroxymethylfurfural (HMF). By measuring levels
> Draw all lone pairs on each of the oxygen atoms in the following structures. Before doing this, review Table 2.2 and then come back to these problems. Try to identify all lone pairs without having to count. Then, count to see if you were correct. From T
> Atenolol and enalapril are drugs used in the treatment of heart disease. Both of these drugs lower blood pressure (albeit in different ways) and reduce the risk of heart attack. Using Table 2.1, identify and label all functional groups in these two compo
> Certain compounds that are alternatives to fossil fuels can be produced by engineered microbes. Bisabolane, an example of a renewable biofuel, is a synthetic alternative to diesel fuel. Draw a bond-line structure for bisabolane: H H H'V H H-C H-C CH(
> Consider the following two isomeric alkenes. The first isomer is a monosubstituted alkene, while the second isomer is a disubstituted alkene. We might expect the second isomer to be more stable, yet heats of combustion for these two compounds indicate th
> Draw a bond-line structure for each of the following compounds: d. (CH3)3C−C(CH3)3 e. CH3CH2CH(CH3)2 f. (CH3CH2)3COH g. (CH3)2CHCH2OH h. CH3CH2CH2OCH3 i. (CH3CH2)2C=CH2 j. CH2=CHOCH2CH(CH3)2 k. (CH3CH2)2CHCH2CH2NH2 l. CH2=CHCH2
> Initially approved to treat psoriasis (a skin disorder) and rheumatoid arthritis, the drug tofacitinib has recently been found to also promote hair growth and restore hair loss. Identify the number of carbon atoms in tofacitinib, and then fill in all of
> For each of the following molecules, determine the number of carbon atoms present and then determine the number of hydrogen atoms connected to each carbon atom: (а) (Ь) (с) (d) (е) (f)
> Oxygenated hydrocarbons (compounds containing carbon, hydrogen, and oxygen) are common components of biofuels. The combustion of three isomers of C3H6O (shown below) were studied to investigate the effects of structure on combustion speed and efficiency.
> Draw a Lewis structure for each of the compounds below: a. CH2=CHOCH2CH(CH3)2 b. (CH3CH2)2CHCH2CH2OH c. (CH3CH2)3COH d. (CH3)2C=CHCH2CH3 e. CH2=CHCH2OCH2CH(CH3)2 f. (CH3CH2)2C=CH2 g. (CH3)3CCH2CH2OH h. CH3CH2CH2CH2CH2CH3 i. CH3CH2CH2OCH3 j. (CH3
> The following structure shows promise for studying how enzymes (nature's catalysts) coil up into very discrete shapes that endow them with catalytic function: a. This compound has two N−C−N units, with differing bond
> Positron emission tomography (PET) is a medical imaging technique that produces a threedimensional picture of functional processes in the body, such as the brain uptake of glucose. PET imaging requires the introduction of [18F]-fluorine (a radioactive is

