Question: Compare the structures of 1,4-pentadiene
Compare the structures of 1,4-pentadiene and divinylamine:
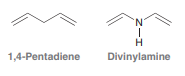
The first compound does not absorb UV light in the region between 200 and 400 nm. The second compound does absorb light above 200Â nm. Using this information, identify the hybridization state of the nitrogen atom in divinylamine and justify your answer.
Transcribed Image Text:
'N' 1,4-Pentadiene Divinylamine Z-I
> Rippertenol is a compound made by termites and used by the insects to defend themselves from predators. Due to its complexity, the first synthesis of rippertenol was not reported until more than 30 years following its original isolation and characterizat
> Predict the major product obtained when each of the following compounds is treated with Birch conditions: (a) (b) (c) HO. HO (d) (e) (f)
> Hamigeran B and several related natural products were isolated from a sponge found off the coast of New Zealand. Hamigeran B has demonstrated strong activity against the viruses that cause polio and herpes. During efforts to synthesize this natural produ
> Propose an efficient synthesis for each of the following transformations: (a) он (Ь) (c) (d) OH (e) (f)
> Draw the expected product when each of the following compounds is oxidized with chromic acid: (a) (b) (c)
> Meclizine, shown below, is an antiemetic (prevents nausea and vomiting). a. Would you expect meclizine to be an antihistamine as well? Justify your answer. b. This drug is known to cause sedation. Describe the source of the sedative properties of mecli
> Identify which compound is expected to have a lower pKa. Justify your choice. N-H
> Draw a mechanism for the following transformation: NaOH, heat
> Draw the two possible enols that can be formed from 3-methyl-2-butanone and show a mechanism of formation of each under base-catalyzed conditions.
> Go to the beginning of Section 17.1 where the structures of several best-selling drugs were shown. Review the structures of those compounds and identify all of the aromatic rings that are not already highlighted in red.
> For each of the following compounds determine which (if any) lone pairs are participating in aromaticity: :N-H (a) (b) (c) (d) (e) (f) (h)
> In a pioneering study on the limits of aromaticity, the following resonance-stabilized dianion was prepared and examined. Predict whether this dianion is aromatic, nonaromatic, or antiaromatic. A etc.
> Determine whether each of the following ions is aromatic, nonaromatic, or antiaromatic: (a) 스 (b) (c) (d)
> Predict whether the following compound will be aromatic, nonaromatic, or antiaromatic. Explain your reasoning.
> The cyclopropenyl cation has a three-membered ring that contains a continuous system of overlapping p orbitals. This system contains a total of two π electrons. Using a Frost circle, draw an energy diagram showing the relative energy levels of
> Predict whether each of the following compounds should be aromatic: (a) (b) (c)
> In some circumstances, dehydrogenation is observed. Dehydrogenation involves the loss of two hydrogen atoms (the reverse of hydrogenation). Analyze each of the following dehydrogenation reactions and then use the information in Figure 17.1 to predict whe
> Compound A is an aromatic compound with the molecular formula C8H8. When treated with excess Br2, compound A is converted into compound B, with the molecular formula C8H8Br2. Identify the structures of compounds A and B. Compound B Compound A (C3H3)
> Aromatic compounds are commonly found in plant oils. For example, an extract of rosemary, which shows anti-oxidant activity, was found to contain the compounds eugenol, thymol, and carvacrol.1 These names are common names; however, each of these compound
> In an effort to make lasers more affordable and easier to make, chemists are developing organic solid-state lasers (OSSLs) using small organic molecules that are capable of lasing (releasing laser light). One such molecule, DPHP, was prepared using an al
> Provide at least five different acceptable IUPAC names for the following compound.
> For each of the following compounds, draw its structure: a. 2,6-Dibromo-4-chloroanisole b. meta-Nitrophenol
> Aromatic compounds often have multiple names that are all accepted by IUPAC. Provide three different systematic (IUPAC) names for the following compound. Br. CH3 HO,
> Provide a systematic name for each of the following compounds: OH Br. Br CH, O2N NO2 Br H. (a) (Ь) (c) он (d) (e)
> Rank the following dienes in terms of reactivity in Diels– Alder reactions (from least reactive to most reactive):
> Consider the following two isomers of 2,4-hexadiene. One isomer reacts rapidly as a diene in a Diels–Alder reaction, and the other does not. Identify which isomer is more reactive and explain your choice. F-/ (2E,4E)-Hexadiene (22,4
> A hetero Diels–Alder reaction is a variation of the Diels–Alder reaction in which one or more of the carbon atoms of the diene and/or the dienophile are replaced by other atoms such as oxygen or nitrogen. With this in
> Predict the products for each of the following reactions: OMe .COOH OMe (a) (Ь) COOH (c) NC-E-CN (d) (e) (f) HO. (h) (i)
> What monomer should be used to make each of the following polymers? F (a) (b) ZEO
> β-Damascenone belongs to a family of fragrant natural products called rose ketones that have been used in the perfume industry. In one synthesis of β-damascenone, the reaction of compound 1 with one equivalent of MCPBA afforded tw
> Identify reagents that can be used to produce each of the following compounds via an aldol reaction. он OH (а) (b) (с) (d) (е) HO.
> Consider the following two dienes. When treated with HBr, one of these dienes yields four products, while the other diene yields only two products. Explain.
> Predict the products for each of the following reactions and propose a mechanism that explains the formation of each product: ? HBr ? HCI HCI (a) (b)
> Draw an energy diagram showing the relative energy levels of the MOs for 1,3,5,7-octatetraene and identify the HOMO and LUMO for both the ground state and the excited state.
> Compare the following three isomeric dienes: a. Which compound will liberate the least heat upon hydrogenation with 2 mol of hydrogen gas? Why? b. Which compound will liberate the most heat upon hydrogenation with 2 mol of hydrogen gas? Why?
> Rank the three C−C bonds of 1-butene in terms of bond length (from shortest to longest).
> As shown, compound 1 can be transformed to compound 2, which undergoes a thio-Claisen rearrangement (similar to a Claisen rearrangement, but with sulfur instead of oxygen) to give compound 3. a. Identify the structure of compound 2, which co
> Roquefortine C belongs to a family of natural products first isolated from cultures of the fungus Penicillium roqueforti in the mid-1970s. Since then, roquefortine C has also been found in other natural sources, including blue cheese. Curiously, isoroque
> Endiandric acids are a class of natural products isolated from the Australian plant Endiandra introrsa. Natural products containing chiral centers are generally found in nature as a single enantiomeric form (optically active), but the endiandric acids ar
> When tricyclic cyclooctyne derivative A reacts with benzyl azide (C6H5CH2N3), a [3+2] cycloaddition occurs between the alkyne and the azide (called a click reaction) to install a new five-membered heterocycle: a. Propose a plausible mechanism for the fo
> During a recent investigation into the chemistry of oligofurans (polymers of the furan heterocycle, which show potential to be used in materials), the investigators observed an interesting reaction with dienophiles. When trifuran (below) is treated with
> Perhydroazulene derivatives have shown usefulness in electronic display applications. In a study to find an efficient route for the synthesis of the perhydroazulene skeleton, compound 1 was heated in an aqueous basic solution. The resulting intramolecula
> Identify reagents necessary to convert cyclohexane into 1,3-cyclohexadiene. Notice that the starting material has no leaving groups and cannot simply be treated with a strong base. You must first introduce functionality into the starting material (for he
> For each of the following compounds, identify whether each C=C Ï€ system is cumulated, conjugated, or isolated: но -OH он О cis-Aconitic acid Plays a role in the citric acid cycle Ocimene Present in the essential ols (a) (b) of many plants
> Numerous herbicides and fungicides are known to contain an acetylenic group. For example, compound A is a pyrimidinone herbicide that functions by inhibiting the accumulation of both chlorophyll and β-carotene. During a synthesis of A, shown
> Fluorenyldiene 1 is a highly conjugated, planar molecule that is readily oxidized to dication 2. Draw at least four other resonance structures of dication 2 and identify why the resonance structure shown here is the greatest contributor to the overall r
> In a mass spectrometric study of nitrogenous aromatic compounds, a peak at m/z = 39 was found for 17 of the 20 compounds investigated, including the 4 compounds in the first row below, with a relative intensity ranging from 5 to 84% of the base peak. Thi
> The following compound, isolated from a New Zealand sea squirt, demonstrates activity against a malarial strain that is resistant to other treatments. Consider the relative basicity of each nitrogen atom in this structure and draw the product expected wh
> When the following cyclopentadiene-fused [14]annulene is treated with KH, a green solution of a stable cyclopentadiene anion results. This transformation causes a dramatic change in part of the 1HÂ NMR spectrum: the methyl groups shift from &a
> Based on your answer to Problem 16.67, propose a mechanism for the following transformation: Heat + CO2
> Propose a mechanism for the following transformation: Heat H.
> Draw the condensation product obtained when each of the following compounds is heated in the presence of aqueous sodium hydroxide: H. -H (a) H. (b) (c) (f)
> Upon irradiation, some organic compounds undergo a reversible change in molecular structure, such as cis–trans isomerization or cyclization of π-conjugatedchromophores. Thisproperty, called photochromism, has shown strong pot
> The poison gelsemoxonine can be isolated from the leaves of a plant native to southeastern Asia (Gelsemium elegans). A key step in a synthesis of this natural product involves a thermally initiated (70°C) sigmatropic rearrangement of the compo
> During studies directed toward the synthesis of atropurpuran, a diterpene with interesting molecular architecture, the investigators used high temperature to convert an acyclic tetraene into a tricyclic compound, shown below. This transformation is belie
> During the first total synthesis of chelidonine, a cytotoxic natural product isolated from the root of Chelidonium majus, the investigators used only heat to achieve the following transformation.7 This transformation is believed to occur via two successi
> Predict the major product of the following Diels–Alder reaction, making sure to consider the regiochemical outcome as well as the stereochemical outcome. ?
> Propose a plausible mechanism for the following transformation. (Hint: Only two sequential pericyclic reactions are required.) Heat
> Would you expect nitroethylene to be more or less reactive than ethylene in a Diels–Alder reaction? (Hint: Draw the resonance structures of nitroethylene.) Ethylene Nitroethylene
> Starting with 1,3-butadiene as your only source of carbon atoms and using any other reagents of your choice, design a synthesis of the following compound: H. En CH3
> Draw all possible conjugated dienes with the molecular formula C6H10, taking special care not to draw the same compound twice.
> Compound A (C7H10) exhibits a λmax of 230 nm in its UV absorption spectrum. Upon hydrogenation with a metal catalyst, compound A will react with two equivalents of hydrogen gas. Ozonolysis of compound A yields the following two compounds. Id
> When conducted in a chiral environment (for example, by using a chiral catalyst or adding a chiral auxiliary), an asymmetric aldol reaction can occur that favors the production of a single stereoisomer over others. The aldehyde shown below was used to di
> α-Terpinene is a pleasant-smelling compound present in the essential oil of marjoram, a perennial herb. Upon hydrogenation with a metal catalyst, α-terpinene reacts with two equivalents of hydrogen gas to produce 1-isopropyl-4
> Cholesta-4,6,8(14)-triene, shown below, was isolated from the thigh gland secretion of the male Great Basin Collared Lizard Crotaphytus bicinctores and is believed to be used for communication.6 Using Woodward–Fieser rules, estimate the
> Propose a mechanism for the following transformation:
> Predict the product(s) obtained when benzoquinone is treated with excess butadiene: O: (Excess) +
> Predict the major product for each of the following reactions: ? Heat (a) ? (c) Heat Heat (b)
> When trans-3,4-dimethylcyclobutene is heated, conrotatory ring opening can produce two different products, yet only one is formed. Draw both products, identify which product is formed, and then explain why the other product is not formed.
> Predict which side of the following equilibrium is favored and explain your choice. Heat
> Identify whether the product obtained from each of the following reactions is a meso compound or a pair of enantiomers: a. Irradiation of (2E,4Z,6Z )-4,5-dimethyl-2,4,6-octatriene with UV light. b. Subjecting (2E,4Z,6Z )-4,5-dimethyl-2,4,6-octatriene t
> Predict the product for each of the following electrocyclic reactions: ? Heat hv (a) (b) ? ? Heat hv (c) (d)
> Propose a plausible mechanism for the following transformation: D Heat
> When each of the following ketones is treated with aqueous sodium hydroxide, the aldol product is obtained in poor yields. In these cases, special distillation techniques are used to increase the yield of aldol product. In each case, predict the aldol ad
> When 5-deutero-5-methyl-1,3-cyclopentadiene is warmed to room temperature, it rapidly rearranges, giving an equilibrium mixture containing the original compound as well as two others. Propose a plausible mechanism for the formation of these other two com
> Predict the expected λmax of the following compound:
> Which of the following compounds do you expect to have a longer λmax?
> Rank the following compounds in order of increasing λmax:
> Cyclopentadiene reacts very rapidly in Diels–Alder reactions. In contrast, 1,3-cyclohexadiene reacts more slowly and 1,3-cycloheptadiene is practically unreactive. Can you offer an explanation for this trend?
> Chlordane is a powerful insecticide that was used in the United States during the second half of the twentieth century. Its use was discontinued in 1988 in recognition of its persistence and accumulation in the environment. Identify the reagents you woul
> The following triene reacts with excess maleic anhydride to produce a compound with the molecular formula C14H12O6. Draw the structure of this product (ignoring stereochemistry). Maleic anhydride (excess)
> Identify the reagents you would use to prepare each of the following compounds via a Diels–Alder reaction: CN .COOH CN ČN CHO (a) 'COOH (ь) ČN (с) CHO (d) (e) .COOH + En (g) ČOOH (h)
> Predict the products for each of the following Diels–Alder reactions: HOOC COOH HOOC CN (a) (Ь) (c) (d) CN (е) NC Meo OMe (f)
> The absorbance spectrum of 1,3-butadiene displays an absorption in the UV region (λmax=217 nm), while the absorption spectrum of 1,2-butadiene does not display a similar absorption. Explain.
> Predict the major product obtained when each of the following aldehydes is treated with aqueous sodium hydroxide: (a) (Ь) (c) (d)
> Rank the following dienophiles (from least reactive to most reactive) in terms of reactivity in a Diels–Alder reaction:
> The compound fusarisetin A (isolated from a soil fungus) displays significant anticancer activity without detectable cytotoxicity (toxicity to cells). A key step in a reported synthesis of the enantiomer of fusarisetin A involves a Dieckmann cyclization
> The following sequence, beginning with a cyclic hemiacetal (compound A), was part of a recently reported enantiospecific synthesis of a powerful sex pheromone (currently used in pest management) of the mealybug Pseudococcus viburni: a. Draw the structur
> The natural product (–)-N-methylwelwitindolinone C isothiocyanate is isolated from a blue-green algae and has been identified as a promising compound to treat drug-resistant tumors. Compound X (R = protecting group) was utilized as a ke
> Draw the major product expected when 1,3-butadiene is treated with one equivalent of HBr at 40ºC, and show a mechanism of its formation.
> Draw the major product expected when 1,3-butadiene is treated with one equivalent of HBr at 0ºC, and show a mechanism of its formation.
> Identify the structure of the conjugated diene that will react with one equivalent of HBr to yield a racemic mixture of 3-bromocyclohexene.
> 113. What enolate is formed in this reaction? 114. Which compounds will react with each other in the presence of NaOH to give the following product? 115. Which set of reagents can be used to achieve this transformation? ? (a) OH OH (다) (d) (a)
> In a recent effort to devise a new synthetic pathway for the preparation of useful building blocks for the synthesis of natural products, compound 2 was prepared by treating compound 1 with diethylmalonate in the presence of potassium carbonate. Propose
> The following synthetic step was utilized as part of a recent synthesis of the polycyclic natural product haouamine B. In this reaction, the function of the first reagent (triflic anhydride) is to activate the Michael acceptor that is present in the star
> Identify reagents that can be used to accomplish each of the following transformations (you will need to use reactions from previous chapters): он OEt (a) CI (b) COOH (c) NH2 (d)
> Predict the product of the following reaction sequence, which was recently used in a synthetic route toward a series of 1,3,6-substituted fulvenes: 1) NaOH/H,O, heat C„H14 2) PhMgCI, THE 3) conc. H2SO4
> Consider the reaction between cyclohexanone and the optically pure amine shown (next page). a. Draw the structure of the resulting enamine and discuss how many isomer(s), if any, form in this reaction. b. When the enamine(s) formed in part
> We have seen that the alpha carbon atom of an enamine can function as a nucleophile in a Michael reaction, and in fact, enamines can function as nucleophiles in a wide variety of reactions. For example, an enamine will undergo alkylation when treated wit
> Using ethanol as your only source of carbon atoms, propose a synthesis for each of the following compounds: OEt (a) (Б) но HO.

