Question: Consider the structure of the following compound:
Consider the structure of the following compound:
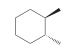
a. When this compound is treated with bromine under conditions that favor monobromination, two stereoisomeric products are obtained. Draw them and identify whether they are enantiomers or diastereomers.
b. When this compound is treated with bromine under conditions that favor dibromination, three stereoisomeric products are obtained. Draw them and explain why there are only three products and not four.
Transcribed Image Text:
> Draw the propagation steps that achieve the autooxidation of diethyl ether to form a hydroperoxide: OOH Diethyl ether A hydroperoxide
> Identify all products expected for each of the following reactions. Take stereochemistry into account and draw expected stereoisomer(s), if any: NBS, hv ? NBS hv ? (a) (b)
> Odor is one of the principal criteria used by consumers when purchasing food products such as fish. Researchers thus compared relative amounts of various volatile substances present in samples of European freshwater catfish raised in an indoor concrete p
> Kakkon is the root of an Asian plant that has traditionally been used in Chinese medicine. It smells fruity, winey, and medicinal. A study of the volatile components of kakkon (those responsible for the scent) resulted in the identification of 33 compoun
> In a study of volatile compounds extracted from cinnamon trees native to Sri Lanka, 27 compounds were identified, including3-phenylpropyl acetate. Propose a multiple-step synthesis of this compound using toluene and any two-carbon reactants as your only
> Starting with acetylene as your only source of carbon atoms, propose a plausible synthesis for 1,4-dioxane: 1,4-Dioxane
> Starting with acetylene as your only source of carbon atoms, identify how you would prepare each member of the following homologous series of aldehydes: a. CH3CHO b. CH3CH2CHO c. CH3CH2CH2CHO d. CH3CH2CH2CH2CHO
> Identify reagents that you could use to achieve the following transformation: Br HO En
> In the previous problem, we saw that an acetylide ion can attack a variety of electrophiles. In Chapter 19, we will see that a C=O bond can also function as an electrophile. Consider the following reaction between an acetylide ion (the nucleophile) and a
> Small organic molecules known as plasticizers are added to polymers to make them soft and flexible. Many phthalates commonly used as plasticizers have been banned from use in children’s toys due to their potential for adverse health eff
> In this chapter, we have seen that an acetylide ion can function as a nucleophile and attack an alkyl halide in an SN2 process. More generally speaking, the acetylide ion can attack other electrophiles as well. For example, we will see in Chapter 13 that
> Propose an efficient synthesis for the following transformation (many steps are required): OH но
> Propose an efficient synthesis for each of the following transformations: X-X, Br. Br (а) Br H (b) Br
> Using acetylene as your only source of carbon atoms, design a synthesis of pentanal (Note: pentanal has an odd number of carbon atoms, while acetylene has an even number of carbon atoms): H. エ
> Using acetylene as your only source of carbon atoms, design a synthesis of cis-3-decene:
> Using acetylene as your only source of carbon atoms, design a synthesis of trans-5-decene:
> Propose an efficient synthesis for each of the following transformations: (a) (b) (c) HO. (d) H. (е)
> Using any compounds that contain two carbon atoms or fewer, show a way to prepare a racemic mixture of (2R,3S)- and (2S,3R)-2,3- dihydroxypentane.
> Using any compounds that contain two carbon atoms or fewer, show a way to prepare a racemic mixture of (2R,3R)-2,3- dihydroxypentane and (2S,3S)-2,3-dihydroxypentane.
> Propose an efficient synthesis for each of the following transformations:
> A compound with the molecular formula C7H14O exhibits the following 13C NMR spectra: Several structures are consistent with these spectra. To determine which structure is correct, a 1H NMR spectrum was acquired which exhibits five signals. One of those
> Identify the structure of a compound with the molecular formula C5H12 that exhibits only one kind of proton. That is, all 12 protons are chemically equivalent.
> Identify reagents that you could use to convert 2-bromo2-methylbutane into 3-methyl-1-butyne.
> Using any reagents you like, show a way to convert 1-methylcyclopentene into 3-methylcyclopentene.
> Propose an efficient synthesis for each of the following transformations: OH (a) он (b)
> Using acetylene as your only source of carbon atoms, identify a synthetic route for the production of 1-bromobutane.
> Using acetylene as your only source of carbon atoms, identify a synthetic route for the production of 2-bromobutane.
> Identify reagents that can be used to achieve each of the following transformations: Br CH, H Br Br Br Br- Br, Br Br Br Br Br O=0=0
> Identify reagents that can be used to achieve each of the following transformations: Br OH Br OH HO. OH -Br Br Br
> A study of compounds contained in the gas phase above a range of bacterial strains revealed the presence of 254 unique volatile organic compounds representing a broad range of structural classes. The two isomers below were among the compounds identified.
> Propose an efficient synthesis for each of the following transformations: Br (a) он (Ь) Br Br | (c) (d) он Br. Br (f) он Br En OH (h)
> Latrunculin B is a marine natural product that was first isolated from the Red Sea sponge Latrunculia magnifica. Latrunculins are toxic to eukaryotic cells (cells that have a welldefined nucleus) by disrupting normal cellular structure. In a reported syn
> Determine the structure of an alcohol with the molecular formula C5H12O that exhibits the following signals in its 13C NMR spectra: a. Broadband decoupled: 73.8 δ, 29.1 δ, and 9.5 δ b. DEPT-90: 73.8 δ c. DEPT-135: positive signals at 73.8 δ and 9.5 δ;
> Identify reagents that can be used to achieve each of the following transformations: (a) (b) Br Br (c) (d) Он H. (e) (f)
> As we will see in Chapter 26, steroids are a class of organic compounds containing a specific tetracyclic skeleton (four rings), as seen in the compounds shown below. During efforts2 to synthesize a steroid that was isolated from the Atlantic starfish, t
> Identify reagents that can be used to achieve each of the following transformations: Br (b) (c)
> The isocoumarins are a class of natural products that are structurally related to isocoumarin, shown below. They have been isolated from many sources, including bacteria, fungi, and plants. Artemidin is an isocoumarin isolated from the tarragon plant (Ar
> Propose an efficient synthesis for each of the following transformations: Br Br (a) (b) (c) (d) Br OH (e) (f) OH OH он он Br LOH (g) (h)
> Identify reagents that can be used to accomplish each of the following transformations. If you are having trouble, the reagents for these transformations appear in the review of reactions at the end of Chapter 9, but you should first try to identify the
> Identify reagents that can be used to accomplish each of the transformations shown below. If you are having trouble, the reagents for these transformations appear in the review of reactions at the end of Chapter 8, but you should first try to identify th
> Natural products that contain the N-1,1-dimethyl-2-propenyl group (called an N-reverse prenyl group) often exhibit antitumor or antifungal activity. The synthesis of a particular N-reverse prenylated indole antifungal compound begins with the two steps s
> Roquefortine C belongs to a class of natural products, called roquefortines, first isolated from cultures of the fungus Penicillium roqueforti. Roquefortine C, which is also present in blue cheese, exhibits bacteriostatic activity (it prevents bacteria f
> Compound X has the molecular formula C7H14. Hydrogenation of compound X produces 2,4-dimethylpentane. Hydroborationoxidation of compound X produces a racemic mixture of 2,4-dimethyl1-pentanol (shown below). Predict the major product(s) obtained when comp
> Determine the structure of a compound with the molecular formula C5H10O that exhibits the following broadband-decoupled and DEPT-135 spectra. The DEPT-90 spectrum has no signals. Broadband-decoupled 220 200 180 160 140 120 100 80 60 40 20 DEPT-135 22
> Suggest an efficient synthesis for the following transformation: OH + En Br
> Compound X has the molecular formula C5H10. In the presence of a metal catalyst, compound X reacts with one equivalent of molecular hydrogen to yield 2-methylbutane. a. Suggest three possible structures for compound X. b. Hydroboration-oxidation of com
> Using acetylene and 2-methylpropane as your only sources of carbon atoms, propose a plausible synthesis for CH3COCH2CH(CH3)2. You will need to utilize many reactions from previous chapters.
> How many constitutional isomers are obtained when each of the following compounds undergoes monochlorination? (a) (b) (c) (d) (e) (f) (g) (h)
> The rate at which two methyl radicals couple to form ethane is significantly faster than the rate at which two tert-butyl radicals couple. Offer two explanations for this observation.
> When 2-methylpropane is treated with bromine in the presence of UV light, one product predominates. a. Identify the structure of the product. b. Draw the structure of the expected minor product. c. Draw a mechanism for formation of the major product.
> Draw the products obtained when 3,3,6-trimethylcyclohexene is treated with NBS and irradiated with UV light.
> Predict the major product(s) obtained upon bromination of (S)-3-methylhexane.
> Identify the major product(s) for each of the following reactions. If any of the reactions do not yield a product, indicate “no reaction.” ? ? Br2 hv (a) (b) ? Cl, NBS, hv hv (c) (d) ? ? hv NBS, hv (e) (f)
> The hydronaphthacene ring system consists of five fused six-membered rings, as shown below in compounds 3 and 4. One method for construction of this ring system involves treatment of compound 1 with a Fischer carbene such as 2. Two diastereomeric product
> For each of the products shown in the following reaction, propose a mechanism that explains its formation: Br NBS hv Br-
> Triphenylmethane readily undergoes autooxidation to produce a hydroperoxide: a. Draw the expected hydroperoxide. b. Explain why triphenylmethane is so susceptible to autooxidation. c. In the presence of phenol (C6H5OH), triphenylmethane undergoes auto
> AIBN is an azo compound (a compound with a N=N double bond) that is often used as a radical initiator. Upon heating, AIBN liberates nitrogen gas to produce two identical radicals: a. Give two reasons why these radicals are so stable. b. Explain why the
> When ethylbenzene is treated with one equivalent of NBS and irradiated with UV light, two stereoisomeric compounds are obtained in equal amounts. Draw the products and explain why they are obtained in equal amounts. NBS hv two products
> There are three constitutional isomers with the molecular formula C5H12. Chlorination of one of these isomers yields only one product. Identify the isomer and draw the product of chlorination.
> When isopropylbenzene (cumene) is treated with NBS and irradiated with UV light, only one product is obtained. Propose a mechanism and explain why only one product is formed. Br NBS hv
> Draw all resonance structures of the radical produced when a hydrogen atom is abstracted from the OH group in BHT: он Butylated hydroxytoluene (BHT)
> Compound A has the molecular formula C5H12 and undergoes monochlorination to produce four different constitutional isomers. a. Draw the structure of compound A. b. Draw all four monochlorination products. c. If compound A undergoes monobromination (in
> Consider all of the different types of C−H bonds in cyclopentene and rank them in order of increasing bond strength: H H H
> Draw all resonance structures for each of the following radicals: (a) (b) „CO (d) (e)
> For each of the following compounds, predict the number of signals and location of each signal in a 13C NMR spectrum: (a) H (b) (c) (d) (e) (f) (g) (h) (i) (j)
> There are 20 commonly occurring amino acids from which all proteins are derived (as will be discussed in Chapter 25), although many other less common amino acids have been isolated from natural sources. Valine is one of the 20 common amino acids, and it
> Predict the products for each reaction. In each case, be sure to consider whether a chiral center is being generated, and then draw all expected stereoisomers. HBr ROOR ? HBr ROOR ? (a) (b) HBr ROOR ? HBr ROOR ? (c) (d) HBr ROOR ? HBr ROOR ? (e) (f)
> Compare the structure of vitamin E with the structures of BHT and BHA, and then determine which hydrogen atom is most easily abstracted from vitamin E.
> Most supersonic planes produce exhaust of hot gases containing many compounds, including nitric oxide (NO). Nitric oxide is a radical that is believed to play a role in ozone depletion. Propose propagation steps that show how nitric oxide can destroy ozo
> GABA, or gamma (γ)-amino butyric acid, is a neurotransmitter (a chemical that is used to send signals from one neuron to another) of the mammalian central nervous system. In order to understand how GABA works, conformationally restricted anal
> When 2-methyl-2-butene is treated with NBS and irradiated with UV light, five different monobromination products are obtained, one of which is a racemic mixture of enantiomers. Draw all five monobromination products and identify the product that is obtai
> Predict the products when each of the following compounds is treated with NBS and irradiated with UV light: (a) (b) (c) (d)
> Carbohydrates such as the glucose derivative shown below can serve as useful chiral starting materials for biopolymers and natural product synthesis. Radical bromination of this compound occurs at C1 to give two products. Draw both products, and predict
> Predict the stereochemical outcome of radical bromination of the following alkanes: (a) (b) (c) (d)
> A synthesis of the antibiotic natural product γ-indomycinone involved the halogenation reaction shown below. The major product of the reaction is a monobrominated compound, and a significant amount of a minor, dibrominated compound is also fo
> Neurotransmitters are small molecules that are produced by the body to send messages from one neuron to another. Noradrenaline (also called norepinephrine) and serotonin are neurotransmitters that act in the central nervous system. A synthetic compound c
> Predict the major product obtained upon radical bromination of each of the following compounds: (a) (b) (c)
> Methyl bromide is widely used as a fumigant to prevent the spread of diseases and pests in agricultural products, and it can be prepared from methane via radical bromination. Recently, a new method for preparing methyl bromide was developed, involving ge
> Draw a mechanism for each of the following reactions: a. Chlorination of methylene chloride to produce chloroform b. Chlorination of chloroform to produce carbon tetrachloride c. Chlorination of ethane to produce ethyl chloride d. Chlorination of 1,1
> The genus Aspergillus includes several hundred species of mold, which collectively produce a large number of natural products. Many of these natural products have been found to possess interesting biological properties. Two of these natural products were
> Draw the appropriate fishhook arrows for each of the following radical processes. - + HBr (a) (Ь) Br *CH3 HBr (c) (d) R -R (e) (f)
> The naturally occurring compound (S)-limonene (compound 1 on the right) can be synthetically transformed with radical chemistry into compound 2, which has potent antimalarial activity. 2 Identify the weakest C−H bond in (S)-limonene.
> The C−H bonds shown in red exhibit very similar BDEs, because homolytic cleavage of either bond results in a resonance-stabilized radical. Nevertheless, one of these C−H bonds is weaker than the other. Identify the wea
> Identify the weakest C−H bond in each of the following compounds: (a) (b) (c) (d)
> Stable radicals have been used as biosensors to monitor cellular oxygen levels. One of the first stable radicals to be reported was the triphenylmethyl radical, shown at right. More recently, similar radicals have been prepared in which the hydrogen atom
> 5-Methylcyclopentadiene undergoes homolytic bond cleavage of a C−H bond to form a radical that exhibits five resonance structures. Determine which hydrogen atom is abstracted and draw all five resonance structures of the resulting radic
> A compound with the molecular formula C10H10O4 produces a 1H NMR spectrum that exhibits only two signals, both singlets. One signal appears at 3.9 ppm with a relative integration value of 79. The other signal appears at 8.1 ppm with a relative integratio
> Draw resonance structures for each of the following radicals: (a) (b) (c) (d)
> Rank the following radicals in order of stability: (a) (b)
> The following reaction does not produce the desired product but does produce a product that is a constitutional isomer of the desired product. Draw the product that is obtained and propose a mechanism for its formation: OH OH 1) NaNH, 2) Mel
> A terminal alkyne was treated with NaNH2 followed by propyl iodide. The resulting internal alkyne was treated with ozone followed by water, giving only one type of carboxylic acid. Provide a systematic, IUPAC name for the internal alkyne.
> In the upcoming chapters, we will learn a two-step method for achieving the following transformation. In the meantime, we have already learned reactions that can be used to accomplish this transformation, although more than two steps are required. Identi
> Identify reagents that you would use to achieve the following transformation:
> Draw the structures of compounds A to D: Bra A 1) Excess NaNH2 B 2) H,0 NANH, 2, D (C,H,2)
> Draw the structure of each possible dichloride that can be used to prepare the following alkyne via elimination:
> Propose an efficient synthesis for each of the following transformations: (a) (b) (c)
> Compound A is an alkyne with the molecular formula C5H8. When treated with aqueous sulfuric acid and mercuric sulfate, two different products with the molecular formula C5H10O are obtained in equal amounts. Draw the structure of compound A, and draw the
> Propose a structure that is consistent with each of the following 1H NMR spectra. In each case, the molecular formula is provided. Proton NMR Proton NMR C,H,0 1 ppm 3.5 3.0 2.0 1.5 1.0 ppm (a) 45.1 Integration Values 18.6 19.1 9.1 (b) Integration Val
> An unknown alkyne is treated with ozone (followed by hydrolysis) to yield acetic acid and carbon dioxide. What is the structure of the alkyne?
> 1,2-Dichloropentane reacts with excess sodium amide in liquid ammonia (followed by water workup) to produce compound X. Compound X undergoes acid-catalyzed hydration to produce a ketone. Draw the structure of the ketone produced upon hydration of compoun

