Question: For each of the following compounds, identify
For each of the following compounds, identify the expected chemical shift for each type of proton:
Transcribed Image Text:
> At high temperatures, alkanes can undergo dehydrogenation to produce alkenes. For example: This reaction is used industrially to prepare ethylene while simultaneously serving as a source of hydrogen gas. Explain why dehydrogenation only works at high te
> Compound 3 below, called mycoepoxydiene, has been isolated from a marine fungus and has been shown to possess anti-cancer and anti-inflammatory properties. It contains an unusual oxygen-bridged cyclooctadiene skeleton. Both enantiomers of this compound w
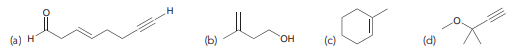
> Identify the reagents you would use to accomplish each of the following transformations: (a) (b) レー人 (c) (d)
> Bioethanol, ethanol produced by fermentation of sugars, is a desirable starting material for chemical synthesis since it comes from renewable resources. Identify the reagents you would use to accomplish the following industrial transformation that conver
> Identify the reagents you would use to accomplish each of the following transformations: он но (a) (b) он Br но En (c) (d) "OH
> In the human body, the amino acid phenylalanine is normally processed by an enzyme that converts it to cinnamic acid. Infants with phenylketonuria (PKU) have a defective enzyme, resulting in a buildup of phenylalanine that can damage a developing brain.
> AZT was the first HIV treatment to be approved by the Food and Drug Administration. HIV may become AZT-resistant over time, so new drugs are always being sought. As part of this effort, compound 2 was made from compound 1 and was shown to have modest act
> Identify the reagents that you would use to accomplish each of the following transformations: Br En (a) (b) Br (c) OH En (e) (f) Br Br | (g) (h)
> Compound A has the molecular formula C5H10. Hydroboration-oxidation of compound A produces a pair of enantiomers, compounds B and C. When treated with HBr, compound A is converted into compound D, which is a tertiary alkyl bromide. When treated with O3 f
> Determine whether syn dihydroxylation of trans-2-butene will yield the same products as anti dihydroxylation of cis-2-butene. Draw the products in each case and compare them.
> Syn dihydroxylation of the compound below yields two products. Draw both products and describe their stereoisomeric relationship (i.e., are they enantiomers or diastereomers?): KMNO4, NaOH ? Cold
> Predict the products of each of the following reactions: 1) вн, - THF 2) H,0, NaOH ? ? PI (a) (Ь) ? :? 1) CH,CO,H 1) OsO, 2) H,0 2) NaHSO,H,O (c) (d) ? HBr ? (е) (f) 1) RCO,H 2) H,0 ? 1) вн, - THF 2) H,O, NaOH ? (g) (h) Оs0, (catalytic) NMO (i)
> Synthetic chemists utilize a large variety of strategies for the synthesis of natural products. Indeed, new strategies are constantly being developed, many of which are inspired by (and mimic) the synthetic pathways employed by nature. As part of the dev
> Identify the structure of the starting alkene in each of the following cases: C3H14 1) Og C10H16 1) O, (a) 2) DMS 2) DMS (b) (c) 1) O 2) DMS
> Predict the products that are expected when each of the following alkenes is treated with ozone followed by DMS: (а) (Ь) (с) (d) (е) (f)
> Predict the product(s) for each of the following reactions. In each case, make sure to consider the number of chiral centers being formed. ? KMno NaOH Oso, (catalytic) NMO 1) Oso, 2) NaHSO, / H,0 ? Cold (al (b) (c) ? Os0, (catalytic) ? ? KMNO,, NaOH
> A compound with the molecular formula C4H6O2 has the following 1H NMR spectrum. Determine the number of protons giving rise to each signal. Proton NMR 5.0 4.5 4.0 3.5 3.0 2,5 2.0 1.5 ppm 18.92 Integration Values 19.77 19.46
> Compound A and compound B both have the molecular formula C6H12. Both compounds produce epoxides when treated with a peroxy acid (RCO3H). a. The epoxide resulting from compound A was treated with aqueous acid (H3O+) and the resulting diol had no chiral
> Under acid-catalyzed conditions, epoxides can be opened by a variety of nucleophiles other than water, such as alcohols. In such a case, the nucleophile will generally attack at the more substituted position. Using this information, predict the products
> Predict the products that are expected when each of the following alkenes is treated with a peroxy acid (RCO3H) followed by aqueous acid: (f)
> When trans-1-phenylpropene is treated with bromine, some syn addition is observed. Explain why the presence of a phenyl group causes a loss of stereospecificity. Br Br + En + En Br Br trans-1-Phenylpropene anti addition products (83%) syn addition pr
> Bromonium ions can be captured by nucleophiles other than water. Predict the products of each of the following reactions: ? Bra EINH, ? HO. (a) (b)
> Predict the major product(s) that are expected when each of the following alkenes is treated with Br2/H2O: (a) (b) (c) (d)
> Predict the major product(s) for each of the following reactions: „I - ? , - ? ? ? Br (b) (c)
> Compound 1 has been shown to be a useful precursor in the synthesis of natural products. In principle, four stereoisomers are possible when this compound is subjected to catalytic hydrogenation. Draw these stereoisomers and describe their relationships:
> Predict the product(s) for each of the following reactions: ? ? Ni Ni (a) (Ь) (c) ? H2 Pt Pd (d) (e)
> α-Pinene can be isolated from pine resin and is a primary constituent of turpentine (paint thinner). Both enantiomers of α-pinene are naturally occurring. To determine the enantiomeric excess (% ee) of α-pinene i
> A compound with the molecular formula C10H10O has the following 1 H NMR spectrum. Determine the number of protons giving rise to each signal. Proton NMR 10 6 5 1 ppm 17.1 87.1 18.7 Integration Values 51.1
> Predict the product(s) for each of the following transformations:
> Compound A has the molecular formula C5H10. Hydroborationoxidation of compound A produces 2-methylbutan-1-ol. Draw the structure of compound A:
> Below are several examples of hydroboration-oxidation. In each case, consider the expected regioselectivity and then draw the product:
> In the first step of oxymercuration-demercuration, nucleophiles other than water may be used. Predict the product for each of the following cases, in which a nucleophile other than water is used.
> Predict the product for each reaction, and predict the products if an acid-catalyzed hydration had been performed rather than an oxymercuration-demercuration:
> If an alkene is protonated and the solvent is an alcohol rather than water, a reaction takes place that is very similar to acid-catalyzed hydration, but in the second step of the mechanism the alcohol functions as a nucleophile instead of water. Draw a m
> Draw a mechanism for each of the following transformations:
> Identify whether you would use dilute sulfuric acid or concentrated sulfuric acid to achieve each of the following transformations. In each case, explain your choice.
> In each of the following cases, identify the alkene that is expected to be more reactive toward acid-catalyzed hydration.
> Carbocation rearrangements during hydrohalogenation reactions can involve shifts of carbon atoms other than a methyl group. For example, the addition of HBr to compound 1 leads to carbocation 2, which rearranges to carbocation 3, before being converted i
> A compound with the molecular formula C5H10O2 has the following 1 H NMR spectrum. Determine the number of protons giving rise to each signal.
> Draw a mechanism for each of the following transformations:
> Predict the products for each of the following reactions. Note: in some cases, the reaction produces a new chiral center, while in other cases, no new chiral center is formed.
> The bicyclo[3.1.0]hexane ring system, highlighted in compound 3, is found in several natural products, including sabinene, a compound partially responsible for the flavor of ground black pepper. One method for preparing this ring system involves the conv
> Draw a mechanism for each of the following transformations:
> Identify the reagents that you would use to achieve each of the following transformations:
> Draw the expected major product for each of the following reactions:
> Reserpine can be isolated from the extracts of the Indian snakeroot Rauwolfia serpentina and has been used in the effective treatment of mental disorders. R. B. Woodward employed the following reaction sequence in his classic 1958 synthesis of reserpine.
> Propose a plausible mechanism for the following transformation, which was used as the key step in the formation of the natural product heliol.
> 9-Borabicyclo[3.3.1]nonane (9-BBN) is a reagent commonly used in the hydroboration of alkynes (Section 10.7), but it can also be employed in reactions with alkenes. The following table provides the relative rates of hydroboration (using 9-BBN) for a vari
> The following table provides relative rates of oxymercuration for a variety of alkenes with mercuric acetate. Provide structural explanations for the trend observed in the relative rates of reactivity. Alkenes…………………………Relative Reactivity CH2C(CH3)(CH2C
> For each of the following compounds, determine whether the two protons shown in red are homotopic, enantiotopic, or diastereotopic:
> John Dewey is the husband of Mary Dewey. He is also the CEO of a large public relations firm. Mary recently filed for divorce, alleging mental brutality, and is asking for half of John's and the couple's assets. In the six months prior to being
> Download and install a network sniffer application like Wireshark, tcpdurop. Sniff the traffic on your local network for 10 minutes and report on your experience. 1. What did you find? 2. Why do these applications exist? 3. How does their existence
> In the fall of 2001, Enron, the eighth largest corporation in the United States, declared bankruptcy unexpectedly, and investors lost approximately $60 billion. From your reading about this famous case, did Enron's bankruptcy involve fraud? If so, what t
> As the new intern for the summer, you have been asked to investigate two methods of e-mail encryption: S/MIME and Pretty Good Privacy (PGP). Compare and contrast the two systems. 1. Why do two standards exist? 2. Which do you think your employer should
> Your company, ABC Reading, writes unique OpenGLbased reading software for children in grade school. ABC employs about 30 sales representatives who interact with school districts around the nation to sell and support your software. ABC has given each sale
> Colleen Matthews had just turned 22 when her hard work finally started to pay off. Six months earlier, Colleen had graduated from a state university with a master's degree in accounting. Colleen graduated with honors and was one of the youngest in her cl
> Dan Jones is the new CIO of Ricochet Systems, an Internet securities broker. After assessing the e-commerce risks in his company, he determines that passwords are a weak link that needs additional protection. However, he is unsure as to what the requirem
> You have been asked by a small credit union to help investigate an alleged fraud in a bankruptcy case in which it is involved. It wants you to start right away because it is worried that the debtor will destroy evidence vital to the case. The credit unio
> John and Sally have recently been experiencing serious marital problems. They are seeing a counselor in order to "save their marriage." During the past several months, John has been selling their recreational assets including an expensive boat, snowmobil
> Bill and Sue were college students when they met each other in the library and began dating. After a few short months, they decided to get married. After a time of marital bliss, both Bill and Sue discovered the relationship was not what they had planned
> 1. Fraud auditors should be equally concerned with liabilities being overstated as well as understated. 2. Proactive searching for analytical symptoms means that we are looking for accounts that appear too high or too low or that are unusual in some ot
> Liz Clayton, supermodel and wife of Andrew Dyce, better known as Flash, lead guitarist and vocalist for the popular heavy metal band Flash Metal, is filing for divorce. Each cited that their careers kept them separated and that they have drifted apart ov
> BBB Company has been a successful manufacturer of quality electronics products for the past 20 years. It is a publicly traded company with I million shares outstanding. During the past three years, the company has fallen on hard times. Profit margins in
> After a jury trial, Charles H. and his parents, Charles M. and Helen J. of White Plains, New York, were found guilty on charges of theft of public funds, wire fraud, bankruptcy fraud, and money laundering conspiracy. The charges arose from the family's s
> One of the riskiest parts of an e-commerce transaction is the payment process. Several different companies, such as Authorize.net, Google, and Yahoo! checkouts, and others provide robust solutions for this risky process. Pick a provider that services the
> eBay has become one of the most popular auction sites in the world. Each day, millions of products and services are bought and sold on the site. Because of its popularity, eBay is also a home for many different types of scams. Your business wants to star
> Your company, ImSecure Inc., is a security investigation firm. You have been contacted by Darling Company, a producer of cardstock for greeting card companies like Hallmike and Birthday Wishes Company. Darling currently requires orders to be placed sever
> Your best friend Sue has always wanted to be an FBI agent for the U.S. government. However, because of the recent restructured changes in the FBI (due to the increased terrorism threat), Sue is uncertain whether she wants to pursue an FBI career. She fee
> You are trying to sell your car. You have been trying to sell it for a while and have it posted on an online classified ad. You receive the following e-mail: Hello. My name is David Meganimus, and I am an assistant to the Greek ambassador to the United S
> Trek, Inc., has experienced two bad financial years, resulting in too few assets remaining to pay creditors in full. Trek, Inc., wants to file for bankruptcy. What are its options, and which one would be best for Trek, Inc.?
> You have been hired as a fraud auditor to examine the assets of a company that recently filed for Chapter 11 bankruptcy. The company manufactures and sells circuit boards for children's computerized toys. You have access to its financial statements and w
> 1. Focusing on changes in financial statements from period to period can help identify analytical fraud symptoms. 2. Controls over inventory should be closely examined when searching for fraud symptoms. 3. The gross profit (margin) ratio is calculate
> Attorney Mark E. of Newport, Virginia, pleaded guilty to wire fraud in the Eastern District of Virginia, based on his actions of embezzling from a client in Chapter 13 bankruptcy. Mark embezzled more than $22,000 intended for payment to the client's mort
> Look up the U.S. Trustee Program's 2012 Annual Report at http://www.justice.gov/ust/eo/public_affairs/ annualreport/docs/ar2012.pdf. 1. How is a trustee defined? 2. What are the functions of the trustee program? 3. The program routinely hires bankrup
> Willy and Buck Forsythe are brothers who often engage in shady business deals and regularly swindle honest people out of their money. Willy and Buck have decided to take their business to a new level. There is a small hardware store in town with a good r
> Steve Stevenson had noticed that the contracts for custodial work for the schools in the district in which he worked had almost all been going to the same custodial company, Johnson Cleaning, and it seemed to just barely manage to be the lowest bidder on
> Hospital administrator Jake Rosen9 was recently convicted for fraud he committed against his employer, Cedar Hospital Systems. Over a period of six years, Jake allegedly made payments to a dummy company for maintenance charges while simultaneously runnin
> You have been searching for a job for some time. One day, while searching through some online want ads, you see the following advertisement: Wanted: Persons seeking high paying corporate jobs in the Cayman Islands. Live the life of your dreams as you wor
> Jenny Lanstrom regularly visits her grandfather, Mike Lanstrom, every Thursday night. Jenny's grandfather has been a widower for the past six years. Jenny's grandfather is very intelligent. He is a decorated veteran of World War II, and over the years, h
> On September 24, 2007, Miguel Carcamo was going through his mail. For some reason, Miguel had not yet received his bank statement, which he usually received at the beginning of each month. Although he was concerned, he took no action and decided not to w
> You work as the assistant to the controller of a small, privately owned company. Part of your job is to create weekly reports of the company's inventories. For the past several months, you have been excluding from your report a room full of damaged and/o
> The following paragraph from the FTC's pamphlet "When Bad Things Happen to Your Good Name" describes the headaches for identity theft victims trying to restore their credit. Unlike victims of other crimes, who generally are treated with respect and symp
> 1. Understated revenues and understated net income are among the most common types of financial statement fraud. 2. Two reasons revenue-related financial statement fraud is so prevalent are because revenue recognition can be highly subjective and becau
> Several years ago, a medical device company was charged with improperly recognizing approximately $1.5 million in revenue from bill-and-hold transactions. One distributor placed orders with the medical device company for a total of approximately 15,000 u
> Introduction Home Safety, Inc.'s management has been trying for months to acquire one of its largest competitors in the home security industry- LockIt-Up Company. Before agreeing to the acquisition, Home Safety's board of directors wanted Lock-It-Up's bo
> For many large, international companies that do business in less developed countries, corruption is a part of everyday life. Without bribing public officials, their companies could never build a factory, hire employees, get permission to build infrastruc
> John is a waiter at a local diner. The diner has a policy that tips are to be pooled between the waiters. Accordingly, each night the cash tips are collected, pooled, and divided up between the various waiters. Then, when employees are paid by the diner,
> Every year, Transparency International makes public its "Corruption Perceptions Index," a measure of how corrupt different countries are in relation to each other. Go to http://cpi.transparency.org/cpi2013/ and learn about the index. 1. Which are some o
> Match the following terms with their corresponding definitions: Billing scheme Asset misappropriation Check tampering Disbursement fraud Expense scheme Investment scam Illegal gratuities Lapping Payroll fraud scheme Skimming 1. Scheme in which
> Hank just loves his new job as a sales clerk at the local classy department store, Fashion's My Style•. It's a great way for him to earn a few dollars while attending high school Not only does his job pay quite well, but it allows him to receive discount
> Regina recently landed her dream job at a local clothes outlet. Within a few weeks of working in her new employment, however, Regina began to engage in fraud. Regina committed the fraud by doing the following: When people returned merchandise, Regina wou
> Jill has recently begun working at a local florist. In addition to creating floral arrangements, Jill spends a good deal of her time talking to customers and ringing up sales. Over time, she identifies a weakness in the procedures for ringing up voids. N
> Ken was the only accountant for a small-town land development company. He was terminated when the company fell on hard times. One year later, when the owner of the company was reviewing the payments received from a landowner for development cost, he disc
> 1. Recording fictitious revenues is one of the most common ways of perpetrating financial statement fraud. 2. Most often, the controller or chief financial officer (CFO) of a corporation is the perpetrator of financial statement fraud because of his or
> In its 2001 annual report, investors of Adelphia Communications were startled to find a footnote in its financial statements that reported the company had guaranteed as much as $2.7 billion in loans to a private entity owned by CEO John Rigas and his fam
> Ed Neilson is the purchasing agent for Style, a nationwide high-fashion women's online store. He joined the company after graduating from college five years ago. Over the years, Ed developed a close relationship with one of the company's vendor's owners-
> In a Las Vegas casino, an employee discovered a flaw in the accounting system. The accounts payable clerk discovered that he was able to change the names of vendors in the computer system to his name. As a result, the employee could create false invoices
> ABCDE Technologies, Inc., designs, manufactures, and markets an extensive line of PC cards. The company sells its PC cards primarily to original equipment manufacturers (OEMs) for industrial and commercial applications in a market with intense competitio
> During the audit of a manufacturing client, you are instructed to do vertical and horizontal financial statement analyses. In your analyses, you notice little increase in the client's overall long-term liabilities. However, you remember that a note was e
> The following two comparative balance sheets and statements of income are for XYZ Company for the years 2015-2017: Calculate all ratios needed to determine if XYZ is possibly underreporting accounts payable. If you detect possible fraud, explain why y

