Question: The following compound is an intermediate in
The following compound is an intermediate in the synthesis of a gelator, which is a compound capable of self-assembling to form a gel in an organic liquid.
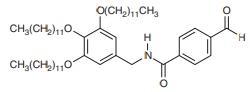
a. Identify each functional group in the molecule.
b. In Chapter 4, we will learn that single bonds experience free rotation at room temperature, while double bonds do not. Consider the two C−N bonds in the structure. One of these bonds exhibits free rotation, as expected for a single bond, but the other C−N bond exhibits restricted rotation. Identify the C−N bond with restricted rotation, and justify your answer by drawing resonance structures.
Transcribed Image Text:
O(CH,)1,CH, CH,(CH,),,0. H H. CH,(CH,),,0
> A total synthesis of the marine natural product aldingenin C in the laboratory resulted in a significant revision of its proposed structure. The synthesis involved the conversion of compound 1 to compound 2, which is an intramolecular SN2-type process. T
> In the presence of a Lewis acid, compound 1 rearranges, via intermediate 2, to afford compound 3. a. Draw curved arrows showing how 1 is transformed into 2. Note that the Lewis acid has been left out for simplicity. b. Draw curved arrows showing how 2
> Compound 1 undergoes a thermal elimination of nitrogen at 250°C to form nitrile 4. One proposed (and subsequently refuted) mechanism for this transformation involves intermediates 2 and 3: a. Draw curved arrows that show the conversion of 2 t
> There are many examples of carbocation rearrangements that cannot be classified as a methyl shift or hydride shift. For example, consider the following rearrangement that was employed in the synthesis of isocomene, a tricyclic compound isolated from the
> The specific rotation of vitamin C (using the D line of sodium, at 20°C) is +24. Predict what the observed rotation would be for a solution containing 0.100 g of vitamin C dissolved in 10.0 mL of ethanol and placed in a sample cell with a length of 1.00
> Identify whether each of the following compounds is chiral or achiral: он (a) (b) (c) (d) OH он (e) Он (f) OH (h) он он (1) (k) (1)
> The specific rotation of (S)-carvone (at +20°C) is +61. A chemist prepared a mixture of (R)-carvone and its enantiomer, and this mixture had an observed rotation of −55°. a. What is the specific rotation of (R)-carvone at 20°C? b. Calculate the % ee of
> Strychnine (6), a notorious poison isolated from the strychnos genus, is commonly used as a pesticide in the treatment of rodent infestations. The sophisticated structure of strychnine, first elucidated in 1946, served as a tantalizing goal for synthetic
> Borane (BH3) is very unstable and quite reactive. Draw a Lewis structure of borane and explain the source of the instability.
> (+)-Aureol is a natural product that shows selective anticancer activity against certain strains of lung cancer and colon cancer. A key step in the biosynthesis of (+)-aureol (how nature makes the molecule) is believed to involve the conversion of carboc
> The following sequence was utilized in a biosynthetically inspired synthesis of preussomerin, an antifungal agent isolated from a coprophilous (dung-loving) fungus. a. Draw curved arrows for each step of the mechanism. b. Include an explanation for the
> Smoking tobacco with a water pipe, or hookah, is often perceived as being less dangerous than smoking cigarettes, but hookah smoke has been found to contain the same variety of toxins and carcinogens (cancer-causing compounds) as cigarette smoke. Draw a
> (S)-2-Iodopentane undergoes racemization in a solution of sodium iodide in DMSO. Explain.
> Compound 1 has been prepared and studied to investigate a novel type of intramolecular elimination mechanism. The proposed mechanistic pathway for this transformation is presented below. Complete the mechanism by drawing curved arrows consistent with the
> Under basic conditions (catalytic MeO− in MeOH), compound 1 rearranges through the mechanistic sequence shown below to a propellane-type isomer 5. Note that MeO− is catalytic in that it is consumed in the first step of
> As seen in Problem 6.18, carbocation rearrangements can occur via the migration of a carbon atom other than a methyl group. For example, carbocation 1 was observed to rearrange to give carbocation 2. a. Identify the carbon atom that is migrating, and dr
> Draw the curved arrows that accomplish the following transformation: H. POH Me Me Me NEN
> When an amine is protonated, the resulting ammonium ion is not electrophilic: However, when an imine is protonated, the resulting iminium ion is highly electrophilic: Explain this difference in reactivity between an ammonium ion and an iminium ion.
> Consider the following reaction. Predict whether an increase in temperature will favor reactants or products. Justify your prediction. ||
> In the presence of a special type of catalyst, hydrogen gas will add across a triple bond to produce a double bond: The process is exothermic. Do you expect a high temperature to favor products or reactants? Catalyst H2
> Draw a Lewis structure for each of the following compounds: a. C2H6 b. C2H4 c. C2H2 d. C3H8 e. C3H6 f. CH3OH
> Identify whether each of the following factors will affect the rate of a reaction: a. Keq b. ΔG c. Temperature d. ΔH e. Ea f. ΔS
> When (R)-3-bromo-2,3-dimethylpentane is treated with sodium hydroxide, four different alkenes are formed. Draw all four products and rank them in terms of stability. Which do you expect to be the major product?
> Consider the following reaction: The following rate equation has been experimentally established for this process Rate = k[HO−][CH3CH2Br] An energy diagram for this process is shown on the right: a. Identify the two characteristic ar
> Predict whether each of the following carbocations will rearrange. If so, draw the expected rearrangement using curved arrows. (a) (b) (c) (d) (e) (f) (g)
> Draw the transition state for the substitution reaction that occurs between ethyl iodide and sodium acetate (CH3CO2Na).
> Draw curved arrows for each step of the following mechanism: HÖ: H HÖ: H-O: OH HÖ: sÖH :OH -O: H HÖ: H HÖ: ÖH HỘ: -H20
> Draw curved arrows for each step of the following mechanism: H :ÖH HO H H, 7 :ÖH I-o. I-z:
> Draw curved arrows for each step of the following mechanism: H@H. :0-H нӧ. TH. TH. TH. H :ÖH :ÖH HÖ но. HO OH HÖ OH H. H. TH. H
> Draw curved arrows for each step of the following mechanism: OH но. CH,SO, NO2 NO2
> For the following mechanism, identify the sequence of arrow-pushing patterns: :ÖH :ÖH OR H R H. R :ÖR R ÖR ÖR
> For the following mechanism, identify the sequence of arrow-pushing patterns: :0 :OH H R R- R R" R R R R R
> Lithium salts have been used for decades to treat mental illnesses, including depression and bipolar disorder. Although the treatment is effective, researchers are still trying to determine how lithium salts behave as mood stabilizers. a. Draw a Lewis st
> Below are two potential methods for preparing the same ether, but only one of them is successful. Identify the successful approach and explain your choice. NaOMe ONa CH3I
> For the following mechanism, identify the sequence of arrow-pushing patterns: :0: :0: :0: H-ö-H H-ö-H R R R R но. :Cl: H H
> The following two reactions will be explored in different chapters. Yet, they are very similar. Identify and compare the sequences of arrowpushing patterns for the two reactions. Reaction 1 (Chapter 19) он :OH HÖ Reaction 2 (Chapter 17) OH :CI: :OH
> For the following mechanism, identify the sequence of arrow-pushing patterns: он OH OH 0=$=0 H H so,
> For the following mechanism, identify the sequence of arrowpushing patterns: CI CI一AI CI マーる G一マーる
> Draw all constitutional isomers with the molecular formula C4H9Br, and then arrange them in order of: a. increasing reactivity toward an SN2 reaction. b. increasing reactivity toward an E2 reaction.
> Draw the transition state for each of the following SN2 reactions: Br OH NaOH + NaBr (a) (Ь) (c) NaOH OH + Naci Br SH NaSH. + NaBr (d) +
> Each of the following compounds exhibits two electrophilic centers. Identify both centers in each compound. (Hint: You will need to draw resonance structures in each case.) H. A cockroach repellant found in cucumbers Nootkatone (a) (b) Found in grape
> The following hypothetical compound cannot be prepared or isolated, because it has a very reactive nucleophilic center and a very reactive electrophilic center, and the two sites would react with each other rapidly. Identify the nucleophilic center and e
> Rank the three carbocations shown in terms of increasing stability: (a) (b)
> For each of the following reactions identify the arrow-pushing pattern that is being utilized: (a) (b) (다) он H. (d)
> Each of the following compounds can be prepared with an alkyl iodide and a suitable nucleophile. In each case, identify the alkyl iodide and the nucleophile that you would use. (a) он (b) CN (c) (d) SH
> Chlorofluorocarbons (CFCs) are gases that were once widely used as refrigerants and propellants. When it was discovered that these molecules contributed to the depletion of the ozone layer, their use was banned, but CFCs continue to be detected as contam
> Which is the better retrosynthesis for the given target molecule? Explain, and provide a one-step synthesis of the target molecule. Br or Br Target molecule
> In Chapter 3, we will explore the factors that render compounds acidic or basic. Tropolone (1) is a compound that is both fairly acidic and fairly basic. It is acidic because it is capable of losing a proton (H+) to form a relatively stable anion (2), wh
> The following compound is an amino acid derivative (Chapter 25). In solution, molecules of this compound show a tendency to “stick” together, or self-assemble, via a series of intermolecular hydrogen bonds.
> Each of 10 stereoisomeric sugar derivatives can be prepared via a multiple-step synthesis starting from either glucuronolactone 1D or its enantiomer 1L. Depending on the specific series of reactions used, the configuration at carbons 2, 3, and 5 can be s
> The natural product meloscine can be prepared via a 19-step synthesis, featuring the following allene as a key intermediate: a. Draw a Newman projection of the allene when viewed from the left side of the C=C=C unit. Note that in this case t
> Consider the structure of the following ketone: a. Does this compound exhibit rotational symmetry? b. Does this compound exhibit reflectional symmetry? c. Is the compound chiral? If so, draw its enantiomer.
> When a toluene solution of the sugar-derived compound below is cooled, the molecules self-assemble into fibrous aggregates which work in concert with the surface tension of the solvent to form a stable gel. Redraw the structure showing both nonaromatic r
> The all-trans-1,2,3,4,5,6-hexaethylcyclohexane (1) prefers the allequatorial conformation while the all-trans-1,2,3,4,5,6-hexaisopropylcyclohexane (2) possesses a severely destabilized all-equatorial conformation. a. By examining a molecular model of cy
> Draw all three staggered conformations for the following compound, viewed along the C2−C3 bond. Determine which conformation is the most stable, taking into account gauche interactions and hydrogen-bonding interactions. Provide a reason
> Triphenylmethane dyes are among the first synthetic dyes developed for commercial use. A comparison of the structures of these compounds reveals that even small differences in structure can lead to large differences in color.15 The structures of three su
> Provide a synthesis for the target molecule shown below, starting with an alkyl halide of your choice. Show your retrosynthetic analysis, and then provide a complete synthesis, showing all necessary reagents. CN
> The following questions apply to the five compounds in the previous problem. a. Which compound is meso? b. Would an equal mixture of compounds b and c be optically active? c. Would an equal mixture of compounds d and e be optically active?
> Draw bond-line structures using wedges and dashes for the following compounds: CH Et Et H- -OH H- -OH но но но -H H- FOH H- -OH H- он но (a) ČH, (b) Me (c) Me Me Me H- -CI C- -H но но- (d) Me (e) Me
> Determine whether each of the following compounds is optically active or optically inactive: CH3 он Et но H. Ме Me- .CI H- он Me (a) H Me OH H CH, (Ь) (с) CH,CH, H. HO H H- он CH,OH CH, ČH, (d) (е) (f) (9) (h) I
> For each of the following pairs of compounds, determine the relationship between the two compounds: Me Me -CI CI- HO Me -H но (а) Me (b) (с) (d) CH, CH, Et Et H- -OH но- H- OH HO но H- -OH но- -H H- он H- -он но- H- OH но FH (е) ČH, ČH, (f) Me Me
> cis-1,3-Dimethylcyclobutane has two planes of symmetry. Draw the compound and identify both planes of symmetry.
> Each of the following compounds possesses a plane of symmetry. Find the plane of symmetry in each compound. In some cases, you will need to rotate a single bond to place the molecule into a conformation where you can more readily see the plane of symmetr
> (R)-Limonene is found in many citrus fruits, including oranges and lemons: For each of the following compounds identify whether it is (R)-limonene or its enantiomer, (S)-limonene: (a) (b) (c) (d)
> When 0.075 g of penicillamine is dissolved in 10.0 mL of pyridine and placed in a sample cell 10.0 cm in length, the observed rotation at 20°C (using the D line of sodium) is −0.47°. Calculate the specific rotation of penicillamine.
> Draw the enantiomer of each compound in the previous problem.
> Determine the configuration for every chiral center in each of the following compounds: OH он он он но- -H но -H HO H но—н H- OH H- -OH HO H HO H ČH,OH CH,OH CH,OH (a) (b) (c)
> Draw a plausible mechanism for each of the following transformations он Br conc. H2SO4 HBr heat (b) OH Он conc. HSO4 HBr HO. Br (d) heat
> Determine whether each statement is true or false: a. A racemic mixture of enantiomers is optically inactive. b. A meso compound will have exactly one nonsuperimposable mirror image. c. Rotating the Fischer projection of a molecule with a single chira
> For each of the following pairs of compounds, determine the relationship between the two compounds: CI (a) (b) он OH (c) CI (d)
> For each of the following pairs of compounds, determine the relationship between the two compounds: но—н но—н CI H OH но- (a) CH,OH CH,OH (b) 'ci 'CI (c) (d)
> The specific rotation of l-alanine in water (at 25°C) is +2.8. A chemist prepared a mixture of l-alanine and its enantiomer, and 3.50 g of the mixture was dissolved in 10.0 mL of water. This solution was then placed in a sample cell with a pathlength of
> The specific rotation of (S)-2-butanol is +13.5. If 1.00 g of its enantiomer is dissolved in 10.0 mL of ethanol and placed in a sample cell with a length of 1.00 dm, what observed rotation do you expect?
> Predict the value for the specific rotation of the following compound. Explain your answer. он HO.
> You are given a solution containing a pair of enantiomers (A and B). Careful measurements show that the solution contains 98% A and 2% B. What is the ee of this solution?
> Identify the configuration of each chiral center in the following compounds: CI Et OH NH, Me (b) (c) HO H F Et (d) CI (e) Me H- OH Cl HO H (f) Me (g)
> Draw the enantiomer for each of the following compounds: OH (а) (b) (с) H- -OH он он ÇI OH H -OH но (d) ČH, (е) Me Me
> Identify the number of stereoisomers expected for each of the following: Me он CI он Me (а) OH (b) Он (с) (d) (е) Но OH (f)
> Predict the major product for each of the following reactions. OH он ? ? HBr conc. H2SO4 heat (a) (b)
> For each of the following pairs of compounds, determine the relationship between the two compounds: Br (a) (b) Br (c) (d) он он (e) (f) (g) он он (h)
> Carbon is not the only element that can function as a chiral center. In Problem 5.6 we saw an example in which a phosphorus atom is a chiral center. In such a case, the lone pair is always assigned the fourth priority. Using this information, assign the
> Assign the configuration of the chiral center in the following compound:
> Paclitaxel (marketed under the trade name Taxol) is found in the bark of the Pacific yew tree, Taxus brevifolia, and is used in the treatment of cancer: a. Draw the enantiomer of paclitaxel. b. How many chiral centers does this compound possess? он
> Atropine, extracted from the plant Atropa belladonna, has been used in the treatment of bradycardia (low heart rate) and cardiac arrest. Draw the enantiomer of atropine: CH -OH
> Atorvastatin is sold under the trade name Lipitor and is used for lowering cholesterol. Annual global sales of this compound exceed $13 billion. Assign a configuration to each chiral center in atorvastatin: он он но. F
> Bogorol A is a natural product with the potential to fight antibiotic-resistant bacteria. Shown below is an intermediate that was used in a synthesis of bogorol A. Assign the configuration of the alkene unit as either E or Z. R. N. OMe Intermediate i
> For each of the following alkenes, assign the configuration of the double bond as either E or Z: (a) (b) CI (c) (d)
> Determine whether each of the following allenes is chiral or achiral: H3C H3C CC=C CH, *CH3 C=C=c "CH,CH3 C=C=C CH3 (a) H (b) (c) (d) CH3
> Protease inhibitors are a class of anti-viral drugs that have had success in treating HIV/AIDS. The following molecules were synthesized as potential HIV protease inhibitors. Compound 1 was found to be an effective protease inhibitor, while compound 2 wa
> Predict the major and minor products for each of the following reactions. OTs NaOE OTs ? ? NaOH (a) (b) LOTS OTs ? NaH ? NASH (c) (d) CH3 1) TSCI, py 2 BUOK ? 1) TsCI, py 2) NaOE! ? HO, (e) (f)
> Identify the configuration of the chiral center in each of the following compounds: OH CH,OH CH;OH H- (а) -NH2 но но -H Br- CH,OH (b) CH3 (с) ČH,CH, (d) CH3
> A racemic mixture with the constitution shown below was isolated from the Maytenus apurimacensis plant that is used in South American folk medicine. In an effort to find another source of these compounds, all of the stereoisomers with this constitution h
> Draw all possible stereoisomers for each of the following compounds. Each possible stereoisomer should be drawn only once: он OH но OH (a) (b) (c) (d) (е)
> Each of the following molecules has one plane of symmetry. Find the plane of symmetry in each case: (Hint: A plane of symmetry can slice atoms in half.) Me (a) Mе Br (b) HO он Me (с) Me (d) (е) (f) CI
> In the previous problem, one object has three planes of symmetry. Identify that object.
> For each of the following objects determine whether or not it possesses a plane of symmetry: (a) (b) (c) (d) (e) (f)
> In the field of chemical research, one area of interest is the study of how variations in structure, including stereochemical variations, affect the biological activity of natural products. (−)-Lariciresinol, shown on the right, is a na
> Identify whether each of the following pairs of compounds are enantiomers or diastereomers: (a) (Ь) он он он он (c) CI (d) (е)
> Synthetic chemists often employ enzymes to conduct asymmetric syntheses that favor the production of one enantiomer over another. Baker’s yeast was used to convert the diketone shown into alcohol 1, with an ee of 84%. The specific rotat
> The specific rotation of vitamin B7 in water (at 22°C) is +92. A chemist prepared a mixture of vitamin B7 and its enantiomer, and this mixture had a specific rotation of +85. Calculate the % ee of this mixture.

