Question: When 2-iodobutane is treated with a
When 2-iodobutane is treated with a variety of bases in DMSO at 50°C, the percentage of 1-butene formed among total butenes is found to be dependent on the choice of base, as seen in the chart below.
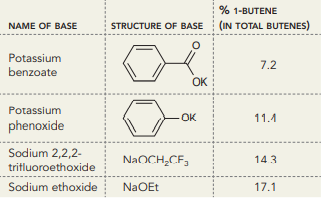
a. Identify the trend observed by comparing the basicity of the bases, and then describe the correlation between reactivity (base strength) and selectivity (specifically regioselectivity).
b. The pKa of 4-nitrophenol is 7.1. Based on the trend in the previous part of this problem, provide an estimate for the percentage of 1-butene that is expected if 2-iodobutane is treated with the conjugate base of 4-nitrophenol in DMSO at 50°C.
Transcribed Image Text:
% 1-BUTENE STRUCTURE OF BASE (IN TOTAL BUTENES) NAME OF BASE Potassium 7.2 benzoate OK Potassium OK 11.4 phenoxide Sodium 2,2,2- NAOCH,CF, 143 trifluoroethoxide Sodium ethoxide NaOEt 17.1
> Draw all constitutional isomers with the molecular formula C5H10 that possess one π bond.
> Propose at least two different structures for a compound with six carbon atoms that exhibits the following features: a. All six carbon atoms are sp2 hybridized. b. Only one carbon atom is sp hybridized, and the remaining five carbon atoms are all sp3 h
> Consider the three compounds shown below and then answer the questions that follow: a. Which two compounds are constitutional isomers? b. Which compound contains a nitrogen atom with trigonal pyramidal geometry? c. Identify the compound with the grea
> Arrange the following compounds in order of increasing boiling point: H H H H H H H H H H H H H H-C, I I I I I H-C-C-c-ċ-ċ-H C-H | ||| | H H H H H-C-C-C-ċ-0-H i iii H H H H H-C C-H H H H H нн H H H HH HH H ||| | H-C-C-Ç-C-c-C-o-H H-C H-C-C-C-H H-C H
> Bonds between carbon and oxygen (C−O) are more polar than bonds between sulfur and oxygen (S−O). Nevertheless, sulfur dioxide (SO2) exhibits a dipole moment while carbon dioxide (CO2) does not. Explain this apparent anomaly.
> Which of the following compounds has the larger dipole moment? Explain your choice: CHCl3 or CBrCl
> Methylene chloride (CH2Cl2) has fewer chlorine atoms than chloroform (CHCl3). Nevertheless, methylene chloride has a larger molecular dipole moment than chloroform. Explain.
> Identify which compounds below possess a molecular dipole moment and indicate the direction of that dipole moment: H. H. H. (a) (b) エ ーエ エ :ö: 0-エ エ H. °C H. H H. :Ci: :Br: (c) (d)
> There are two different compounds with the molecular formula C2H6O. One of these isomers has a much higher boiling point than the other. Explain why.
> Classify each of the following alkenes as monosubstituted, disubstituted, trisubstituted, or tetrasubstituted: (b) (c) (e)
> Below is the structure of caffeine, but its lone pairs are not shown. Identify the location of all lone pairs in this compound: H H c-H H, I !! C-H HIH Caffelne z-0-I エーO I I
> Nicotine is an addictive substance found in tobacco. Identify the hybridization state and geometry of each of the nitrogen atoms in nicotine: H C-H H. .C. C N H H C. H. H Nicotine I I-U-0-I
> Identify the most electronegative element in each of the following compounds: a. CH3OCH2CH2NH2 b. CH2ClCH2F c. CH3Li
> Ambienâ„¢ is a sedative used in the treatment of insomnia. It was discovered in 1982 and brought to market in 1992 (it takes a long time for new drugs to undergo the extensive testing required to receive approval from the Food and Drug Ad
> Identify the hybridization state and geometry of each carbon atom in the following compounds: (a) (b) エ エ エー エ エ O-エ エ エ をー0三 マー三
> For each case below, identify the most likely value for x: a. BHx b. CHx c. NHx d. CH2Clx
> Which of the following pure compounds will exhibit hydrogen bonding? a. CH3CH2OH b. CH2O c. C2H4 d. C2H2 e. CH3OCH3 f. CH3NH2 g. C3H8 h. NH3
> For each pair of compounds below, predict which compound will have the higher boiling point and explain your choice: a. CH3CH2CH2OCH3 or CH3CH2CH2CH2OH b. CH3CH2CH2CH3 or CH3CH2CH2CH2CH3 H H H H O H | ||| H-C-C-C-H or H-C-C-C-H (c) н HH H
> Count the total number of σ bonds and Ï€ bonds in the compound below: H H H-Ö-C-C=c-C=C-ċ-Ñ-H H HH нн
> Identify the expected hybridization state and geometry for the central atom in each of the following compounds: H (a) н H. (b) н- H (c) HeH . (d) H H. H. エーC:
> In Section 4.2, we learned how to name bicyclic compounds. Using those rules, together with the rules discussed in this section, provide a systematic name for the following bicyclic compound. In a case like this, the lowest number is assigned to a bridge
> Predict the bond angles for all bonds in the following compounds: a. CH3CH2OH b. CH2O c. C2H4 d. C2H2 e. CH3OCH3 f. CH3NH2 g. C3H8 h. CH3CN
> For each type of bond below, determine the direction of the expected dipole moment: a. C−O b. C−Mg c. C−N d. C−Li e. C−Cl f. C−H g. O−H h. N−H
> Draw structures for any five constitutional isomers with the molecular formula C2H6O3.
> Draw structures for all constitutional isomers with the following molecular formulas: a. C2H6O b. C2H6O2 c. C2H4Br2
> In the compounds below, classify each bond as covalent, polar covalent, or ionic: a. NaBr b. NaOH c. NaOCH3 d. CH3OH e. CH2O
> Identify the neutral element that corresponds with each of the following electron configurations: a. 1s2 2s2 2p4 b. 1s2 2s2 2p5 c. 1s2 2s2 2p2 d. 1s2 2s2 2p3 e. 1s2 2s2 2p6 3s2 3p5
> Determine whether each compound below exhibits a molecular dipole moment: a. CH4 b. NH3 c. H2O d. CO2 e. CCl4 f. CH2Br2
> Draw the structure for the only constitutional isomer of cyclopropane: H H H-C-C-H Cyclopropane
> Draw a Lewis structure of the anion AlBr4− and determine its geometry.
> Draw a Lewis structure for a compound with the molecular formula C4H11N in which three of the carbon atoms are bonded to the nitrogen atom. What is the geometry of the nitrogen atom in this com pound? Does this compound exhibit a molecular dipole moment
> Draw a bond-line structure for each of the following compounds: a. 3-Isopropyl-2,4-dimethyl-2-pentene b. 4-Ethyl-2-methyl-2-hexene c. 1,2-Dimethylcyclobutene (The name of a cycloalkene will often not include a locant to specify the position of the π b
> Draw a Lewis dot structure for each of the following compounds: a. CH3CH2OH b. CH3CN
> For each pair of compounds below, identify the one that would be expected to have more ionic character. Explain your choice. a. NaBr or HBr b. BrCl or FCl
> For each compound below, identify any polar covalent bonds and indicate the direction of the dipole moment using the symbols δ+ and δ−: a. HBr b. HCl c. H2O d. CH4O
> Draw structures for all constitutional isomers with the molecular formula C4H8 that have: a. Only single bonds b. One double bond
> Draw structures for all constitutional isomers with the following molecular formulas: a. C6H14 b. C2H5Cl c. C2H4Cl2 d. C2H3Cl3
> Epichlorohydrin (1) is an epoxide used in the production of plastic, epoxy glues, and resins (reactions of epoxides will be discussed in Chapter 13). When epichlorohydrin is treated with phenol (2), two products are formed (3 and 4). These two products c
> For each of the following pairs of compounds, identify the higher boiling compound and justify your choice: нн нн H-c-0-c-H Ç-H H-C ハ нн (a) нн H H H VH H-c H .C H H-C C- ーH (b) H H нн C-H Ö-H (c) H-C :ö-H 一H H-C c-H (d) H H H H エ エーOーエ エーOーエ :O: エーO
> Volatile organic compounds (VOCs) contribute to the aroma of plants and can also be used for communication between plants. Diphenyl ether was identified as a minor VOC found in tomato plants. Identify whether diphenyl ether exhibits a molecular dipole mo
> Identify whether each of the following compounds exhibits a molecular dipole moment. For compounds that do, indicate the direction of the net molecular dipole moment: (a) CHCI3 (b) CH3OCH3 (c) NHз (d) CCl,Br2 нн H || H H H H H H-C °C C-H H-C C-H H-C
> When sand is coated with a layer of trimethylhydroxysilane, (CH3)3SiOH, it repels water and can no longer get wet. Hydrophobic sand (aka, magic sand) is fun to play with, but it can also have useful applications in agriculture to reduce water consumption
> Provide a systematic name for each of the following compounds: (a) (b) (c) (d) (e) (g) (h)
> Ammonia (NH3) will react with a strong acid, such as hydronium (H3O+), to give an ammonium ion, as shown below. This type of process is an acid-base reaction, which will be the topic of Chapter 3. Using VSEPR theory, determine whether you expect a change
> Compare the structures of a carbocation and a carbanion: In one of these ions, the central carbon atom is trigonal planar, while the other is trigonal pyramidal. Using VSEPR theory, assign the correct geometry to each ion. Carbocation Carbanion
> Use VSEPR theory to predict the geometry for each of the following structures: H CI le H-B-H CI-C-H H-N-H .B (a) (Ь) F (c) (d) ČI エーZーエ エーローエ
> Rank the indicated bonds in terms of increasing bond length: H H.I c-CEc-H H H c=C H-C-C H H it
> Nemotin is a compound that was first isolated from the fungi Poria tenuis and Poria corticola in the 1940s and was shown to possess potent antibacterial activity. However, its structure was not verified until it was made in the laboratory much more recen
> Determine the hybridization state of each carbon atom in the following compounds. H H H нн ! ! H-C-CEC-C-C-H H H-C-C H-C-C-c. C-H H H H (a) (Ь) H H H (с) H H C=c=c=C (d) н H (е) н"
> Steroids and their derivatives are among the most widely used therapeutic agents. They are used in birth control, hormone replacement therapy, and in the treatment of inflammatory conditions and cancer. New steroid derivatives are discovered regularly by
> Biotin (compound 4) is an essential vitamin that plays a vital role in several important physiological processes. A total synthesis of biotin, developed by scientists at Hoffmann-La Roche, involved the preparation of compound 1. Conversion of 1 to 4 requ
> Halogenated derivatives of toluene will undergo hydrolysis via an SN1 process: The rate of hydrolysis is dependent on two main factors: 1. the stability of the leaving group, and 2. the stability of the intermediate carbocation. The following are rat
> The following reaction exhibits a second-order rate equation: a. What happens to the rate if the concentration of chlorocyclopentane is tripled and the concentration of sodium hydroxide remains the same? b. What happens to the rate if the concentration
> Compound 1 was prepared during a recent synthesis of 1-deoxynojirimycin, a compound with application to HIV chemotherapy. Upon formation, compound 1 rapidly undergoes ring contraction in the presence of chloride ion to form compound 2. Propose a plausibl
> Identify the electron configuration for each of the following atoms: a. Carbon b. Oxygen c. Boron d. Fluorine e. Sodium f. Aluminum
> Plastics and synthetic fibers are examples of the many materials made from repeating subunits of carbon-containing molecules called polymers. Although most synthetic polymers are prepared from fossil fuel sources, many researchers are exploring ways to m
> The regions of δ+ in a compound are the regions most likely to be attacked by an anion, such as hydroxide (HO−). In the compound shown, identify the two carbon atoms that are most likely to be attacked by a hydroxide ion.
> 99. For the following substitution reaction, which statement is FALSE? a. The process is bimolecular. b. Increasing the concentration of hydroxide will cause an increase in the rate of reaction. c. The use of a polar aprotic solvent will enhance the r
> The following sequence of reactions was performed during a synthesis of (+)-coronafacic acid, a key component in the plant toxin coronatine. Predict the product of this reaction sequence, and justify the regiochemical outcome of the second reaction.
> Compound 1 is observed to undergo debromination upon treatment with DMF to afford an alkene. The E-isomer (compound 2) is obtained exclusively (none of the Z-isomer is observed). A concerted mechanism (shown below) has been refuted by the observation tha
> Cyclopropyl chloride (1) cannot generally be converted into cyclopropanol (4) through a direct substitution reaction, because undesired, ring-opening reactions occur. The following represents an alternative method for preparing cyclopropanol. a. Compoun
> The optically pure octyl sulfonate shown below was treated with varying mixtures of water and dioxane, and the optical purity of the resulting product (2-octanol) was found to vary with the ratio of water to dioxane, as shown in the following table. As i
> Thienamycin is a potent antibacterial agent isolated from the fermentation broth of the soil microorganism, Streptomyces cattleya. The following SN2 process was utilized in a synthesis of thienamycin. Draw the product of this process (compound 3). Li
> Choline is a compound involved in neurotransmission. The biosynthesis of choline involves the transfer of a methyl group from SAM. Draw a mechanism for this transformation: CH3 H,C N- OH -он SAM H,C-N- CH3
> Assign a systematic name for each of the following compounds: Br, Br (b) (c) (d) Br CI (e) (f) (h)
> Assign a name for each of the following compounds. Be sure to assign the configuration of each chiral center and indicate the configuration(s) at the beginning of the name. Me (a) (b) (c)
> Assign a name for each of the following compounds: (a) (b)
> Predict the products for each of the following reactions: Excess HBr Excess HBr ? Excess HỊ ? ? Heat Нeat (a) (b) Heat yI? „O^E ? .O Excess HI Excess HBr Excess ? Heat Heat Heat (d) (e) (f)
> Show how you would use an alkoxymercuration-demercuration to prepare isopropyl propyl ether using propene as your only source of carbon and any other reagents of your choosing.
> How would you use an alkoxymercuration-demercuration to prepare dicyclopentyl ether using cyclopentene as your only source of carbon?
> Identify reagents that you could use to prepare each of the following ethers via an alkoxymercuration-demercuration: OEt (a) (b)
> Compound 2 was made as a potential anti-HIV agent, based on its structural similarity to other reported anti-HIV compounds. One of the early steps in the synthesis involved the creation of an ether group (highlighted) in compound 1. Identify reagents tha
> Show reagents that you could use to prepare each of the following ethers via a Williamson ether synthesis and explain your reasoning: -OMe (a) (b) (c) (d)
> Identify the missing reagent needed to achieve each of the following transformations: Br F Br KF NaF benzene benzene ? (a) (b) Br OH OH LIF KMNO, benzene benzene ? ? (c) (d)
> Identify the structure of a compound with the molecular formula C9H20 that exhibits four CH2 groups, all of which are chemically equivalent. How many total signals would you expect in the 1H NMR spectrum of this compound?
> Propose a plausible mechanism for each of the following transformations: OH 1) MeMgBr 2) H,0 (a) 1) Excess MeMgBr 2) H20 но он (Б)
> Diethyl ether was commonly used as an anesthetic before its negative effects on the human nervous system were recognized. As part of a study to develop a method for predicting toxicity based on structure, the observed toxicity of a variety of dialkyl eth
> Draw a structure for each of the following compounds: a. (R)-2-Ethoxy-1,1-dimethylcyclobutane b. Cyclopropyl isopropyl ether
> Provide an IUPAC name for each of the following compounds: .CI (а) (b) (с) он OE! (d) (е)
> Suggest an efficient synthesis for the following transformation: CI H H.
> A variety of phenyl-substituted acetylenes (1a–d) were treated with HCl to give a mixture of E and Z isomers, as shown below a. As we saw in Problem 9.72, vinyl carbocations can form if they are stabilized by resonance. Draw the vinyl
> The two lowest energy conformations of pentane are the anti-anti and the anti-gauche forms, in terms of arrangements around the two central C−C bonds. A recent study analyzed the conformations of 3-heptyne as an “elongated” analogue of pentane, where a c
> Brevetoxin B (compound 2) is produced by Ptychodiscus brevis Davis, a marine organism responsible for red tides. Brevetoxin B is a potent neurotoxin, as a result of its ability to bind to sodium channels and force them to remain open. Its biological acti
> The compound 5,9,12,16-tetramethyleicosane was synthesized as part of a study of the male sex pheromone of a Brazilian bug that feeds on the leaves and fruit of tomato plants. The synthesis of this alkane was reported using compounds Aâ
> Laureatin is a natural product isolated from the marine algae Laurencia nipponica that exhibits potent insecticidal activity against mosquitos. During an investigation toward the synthesis of laureatin, an interesting skeletal rearrangement was observed.
> The following procedure is part of a synthetic strategy for the enantioselective preparation of carbohydrates (Chapter 24): a. Under these strongly basic conditions (NaOH), the alcohol group is deprotonated to give an alkoxide ion, which can then funct
> Predict the products for each of the following: 1) Og 2) DMS 3) Excess LIAIH, 4) H0 ? 1) Os 2) DMS 3) Excess LIAIH, 4) H20 (a) (b) 1) EIMgBr 2) H,0 3) Na, Cr,0, H,SO4, H,0 4) EIMgBr ? (c) 1) LIAIH, 2) H,0 3) TsCI, pyridine ? (d) H 1) Hgo 2) Na, Cr,0,
> How many signals do you expect in the 1H NMR spectrum of each of the following compounds: OH Geraniol Isoprene (b) A precursor for natural rubber Isolated from roses (a) and used in perfumes
> Halogenation of alkynes with Cl2 or Br2 can generally be achieved with high yields, while halogenation of alkynes with 2 typically gives low yields. However, the following reaction is successfully completed with 2 in high yields (94%) to afford a potenti
> Salvinorin A, isolated from the Mexican plant Salvia divinorum, is known to bind with opioid receptors, thereby generating a powerful hallucinogenic effect. It has been suggested that salvinorin A may be useful in the treatment of drug addiction. Termina
> Treatment of one mole of dimethyl sulfate (CH3OSO3CH3) with two moles of sodium acetylide results in the formation of two moles of propyne as the major product: a. Draw the inorganic, ionic species that is generated as a by-product of this reaction and
> Reboxetine mesylate is used in the treatment of depression and is currently marketed as the racemate. The (S,S)-enantiomer of reboxetine is being evaluated for the treatment of neuropathic pain. The following synthetic scheme was part of the synthesis of
> During a total synthesis of 2-methyl-d-erythritol (a sugar of importance to isoprenoid biosynthesis), epoxide 2 was required. a. Identify the reagents you would use to achieve a stereoselective synthesis of epoxide 2 from allylic alcohol 1. b. Propose
> Guggul is an herbal extract from the resin of the mukul myrrh tree, and it shows potential for treating high cholesterol. In a recent synthesis of (+)-myrrhanol A (a compound present in guggul), compound 1 was treated with MCPBA, followed by
> Sphingolipids are a class of compounds that play an important role in signal transmission and cell recognition. Fumonisin B1 is a potent sphingolipid biosynthesis inhibitor. A recent synthesis of fumonisin B1 employed the following transformation, invol
> The following sequence of reactions was employed during synthetic studies on reidispongiolide A, a cytotoxic marine natural product.8 Draw the structures of compounds A, B, C, and D cat. OsO. B Ph 1) CH2=CHMgBr 1) NaH A 2) CHI C + D Ph Ph 2) Н,о NMO
> The SN2 reaction between a Grignard reagent and an epoxide works reasonably well when the epoxide is ethylene oxide. However, when the epoxide is substituted with groups that provide steric hindrance, a competing reaction can dominate, in which an allyli
> Identify the reagents you would use to accomplish each of the following transformations: он он (а) (b) Он но. HO. (c) (d) он он H (е) (f)
> Using bromobenzene and ethylene oxide as your only sources of carbon, show how you could prepare trans-2,3-diphenyloxirane (a racemic mixture of enantiomers). + Enantiomer
> A compound with the molecular formula C9H18 exhibits a 1H NMR spectrum with only one signal and a 13C NMR spectrum with two signals. Deduce the structure of this compound.

