Question: Complete the following table with the nuclear
Complete the following table with the nuclear particle that is produced in each nuclear reaction.
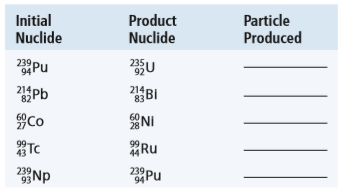
Transcribed Image Text:
Initial Nuclide Product Nuclide Particle Produced 239Pu 94 235 김 Pb BI 214 214 83 Co NI 99- Ru 239 93 239 Pu
> Why must the sum of all the oxidation states of the atoms in a neutral molecule be zero?
> The “pressure” on electrons to flow from one electrode to the other in a battery is called the of the battery.
> In a(n) cell, electrons flow through a wire from the reducing agent to the oxidizing agent.
> What systematic ending is used to show that a molecule is a carboxylic acid? Give an example.
> Which process (oxidation/reduction) takes place at the anode of a galvanic cell?
> An electrochemical cell that produces a current from an oxidation–reduction reaction is often called a(n) cell.
> To obtain useful electrical energy from an oxidation–reduction process, we must set up the reaction in such a way that the oxidation half-reaction and the reduction half-reaction are physically one another.
> When we balance an oxidation–reduction equation, the number of electrons lost by the reducing agent must the number of electrons gained by the oxidizing agent.
> To function as a good reducing agent, a species must electrons easily.
> An oxidizing agent causes the (oxidation/reduction) of another species, and the oxidizing agent itself is (oxidized/reduced).
> What structural feature distinguishes aldehydes from ketones?
> let’s us keep track of electrons in oxidation– reduction reactions by assigning charges to the various atoms in a compound.
> Why is methanol sometimes called wood alcohol? Describe the modern synthesis of methanol. What are some uses of methanol?
> Although nuclear processes offer the potential for an abundant source of energy, no nuclear power plants have been built in the United States for some time. In addition to the fear of a malfunction in such a plant (as happened at the Three Mile Island nu
> How do the chemical properties of radioactive nuclei (as opposed to the nuclear decay they undergo) influence the degree of damage they do to an organism?
> Explain why, although gamma rays are far more penetrating than alpha particles, the latter are actually more likely to cause damage to an organism. Which radiation is more effective at causing ionization of biomolecules?
> Write the equation for the synthesis of ethanol from ethylene. What are some commercial uses of ethanol made by this process?
> The “Chemistry in Focus” segment Water-Powered Fireplace discusses a fireplace that uses the electrolysis of water to produce hydrogen gas. Write the balanced chemical equation for the electrolysis of water. Which element in water is oxidized? Which is r
> Although the energy transferred per event when a living creature is exposed to radiation is small, why is such exposure dangerous?
> What are some important uses of electrolysis?
> What type of “fuel” could be used in a nuclear fusion reactor, and why is this desirable?
> If a benzene ring contains several substituents, how are the relative locations of the substituents numbered in the systematic name given to the molecule?
> For each of the following oxidation–reduction reactions, identify which element is oxidized and which is reduced. a. Mg(s) + Br2( l ) MgBr2(s) b. 2Na(s) + S(s) Na2S(s) c. CO2( g) + H2( g) CO( g) + H2O( g)
> Benzene exhibits resonance. Explain this statement in terms of the different Lewis structures that can be drawn for benzene.
> Explain how some metals, notably aluminum, naturally resist complete oxidation by the atmosphere.
> What is a meltdown, and how can it occur? Most nuclear reactors use water as the cooling liquid. Is there any danger of a steam explosion if the reactor core becomes overheated?
> Can a nuclear explosion take place in a reactor? Is the concentration of fissionable material used in reactors large enough for this?
> Describe the purpose of each of the major components of a nuclear reactor (moderator, control rods, containment, cooling liquid, and so on).
> What does it mean to say that fissionable material possesses a critical mass? Can a chain reaction occur when a sample has less than the critical mass?
> The thyroid gland is interesting in that it is practically the only place in the body where the element iodine is used. How have radiotracers been used to study and treat illnesses of the thyroid gland?
> Why does an ancient wood or cloth artifact contain less 6 14
> In dating artifacts using carbon-14, an assumption is made about the amount of carbon-14 in the atmosphere. What is this assumption? Why is the assumption important?
> What is a salt bridge? Why is a salt bridge necessary in a galvanic cell? Can some other method be used in place of the salt bridge?
> For each of the following oxidation–reduction reactions, identify which element is being oxidized and which is being reduced. a. Ca(s) + 2H2O(l) Ca(OH)2(s, aq) + H2(g) b. H2(g) + F2(g) 2HF( g) c. 4Fe(s) + 3O2(g)
> Describe in general terms how an archaeological artifact is dated using carbon-14.
> The element krypton has several radioactive isotopes. Below are listed several of these isotopes along with their half-lives. Which of the isotopes is most stable? Which of the isotopes is “hottest”? If we were to begin a half-life experiment with separa
> Iodide ion, I-, is one of the most easily oxidized species. Balance each of the following oxidation–reduction reactions, which take place in acidic solution, by using the “half-reaction” method. a. IO3-(aq) + I-(aq) I2(aq) b. Cr2O72-(aq)
> Balance each of the following oxidation–reduction reactions, which take place in acidic solution, by using the “half-reaction” method. a. Al(s) + H+(aq) / Al3+(aq) + H2(g) b. S2–(aq) + NO3–(g) / S(s) + NO(g) c. I2(aq) + Cl2(aq) / IO3–(aq) + HCl(g) d.
> Balance each of the following oxidation–reduction reactions, which take place in acidic solution, by using the “half-reaction” method. a. Mg(s) + Hg2+(aq) / Mg2+(aq) + Hg22+(aq) b. NO3–(aq) + Br–(aq) / NO(g) + Br2(l) c. Ni(s) + NO3–(aq) / Ni2+(aq) + NO
> Balance each of the following half-reactions, which take place in acidic solution. a. O2(g) / H2O(l) b. SO42–(aq) / H2SO3(aq) c. H2O 2(aq) / H2O(l) d. NO2–(aq) / NO3–(aq)
> Balance each of the following half-reactions, which take place in acidic solution. a. HClO(aq) Cl-(aq) b. NO(aq) N2O( g) c. N2O(aq) N2( g) d. ClO3-(aq) HClO2(aq)
> Balance each of the following half-reactions. a. 3N2( g) + 2e- 2N3-(aq) b. O22-(aq) O2( g) c. Zn(s) Zn2+(aq) d. F2( g) F-(aq)
> Balance each of the following half-reactions. a. Cu / Cu2+ b. Fe3+ / Fe2+ c. Br– / Br2 d. Fe2+ / Fe
> Why must the number of electrons lost in the oxidation equal the number of electrons gained in the reduction? Is it possible to have “leftover” electrons in a reaction?
> For each of the following oxidation–reduction reactions, identify which element is oxidized and which is reduced. a. 6Na(s) + N2( g) 2Na3N(s) b. Mg(s) + Cl2( g) MgCl2(s) c. 2Al(s) + 3Br2( l ) 2AlBr3(s)
> What is a half-reaction? What does each of the two half- reactions that make up an overall process represent?
> Why is a systematic method for balancing oxidation–reduction reactions necessary? Why can’t these equations be balanced readily by inspection?
> In what two respects must oxidation–reduction reactions be balanced?
> Potassium iodide in solution reacts readily with many reagents. In the following reactions, identify the atoms that are being oxidized and reduced, and specify the oxidizing and reducing agents. a. Cl2( g) + KI(aq) KCl(aq) + I2(s) b. 2
> Consider the oxidation–reduction reaction Mg(s) + Cu2+(aq) / Mg2+(aq) + Cu(s) Sketch a galvanic cell that uses this reaction. Which metal ion is reduced? Which metal is oxidized? What half-reaction takes place at the anode in the cell? What half-reactio
> Consider a galvanic cell based on the following oxidation– reduction reaction: 2Al3+(aq) + 3Mg(s) ( 2Al(s) + 3Mg2+(aq) What will the electrode found in the cathode portion of the cell be made of? Explain your answer. a. air b. HCl c. Mg d. Al e. H2
> Each of the following nuclides is known to undergo radioactive decay by production of a ß particle, /. Write a balanced nuclear equation for each process. a. 53 136
> Write a nuclear equation showing the bombardment of beryllium-9 with alpha particles, resulting in production of carbon-12 and a neutron.
> Write a balanced nuclear equation for the bombardment of 7 14
> How many of the following statements regarding the decay of radioactive nuclides are true? a. During a given period of time, a radioactive nucleus with a short half-life is much more likely to decay than one with a long half-life. b. As a nuclide decay
> What do we mean when we say that one radioactive nucleus is “hotter” than another? Which element would have more decay events over a given period of time?
> Complete each of the following nuclear equations by supplying the missing particle. a. 88 226
> Aluminum exists in several isotopic forms, including /Al, /Al, and /Al. Indicate the number of protons and the number of neutrons in each of these isotopes.
> The element zinc in nature consists of five isotopes with higher than 0.5% natural abundances, with mass numbers 64, 66, 67, 68, and 70. Write the nuclear symbol for each of these isotopes. How many protons does each contain? How many neutrons does each
> Zirconium consists of five primary isotopes, of mass numbers and abundances shown below: Zr-90………...51.5% Zr-91………….11.2% Zr-92………...17.1% Zr-94……….17.4% Zr-96…………2.8% Write the nuclear symbol,
> Each of the following nuclides is known to undergo radioactive decay by production of a ß particle, /. Write a balanced nuclear equation for each process. a. / b. / c. /
> The fission of 92 235
> Each of the following isotopes has been used medically for the purpose indicated. Suggest reasons why the particular element might have been chosen for this purpose. a. cobalt-57, for study of the body’s use of vitamin B12 b. calcium-47, for study of b
> Supply the missing particle, and state the type of decay for each of the following nuclear processes. a. + ? He b. + ? Pa
> The most common type of nuclear reactor uses the nuclide as its fissionable material.
> are radioactive substances that physicians introduce into the body to enable them to study the absorption and metabolism of the substance or to analyze the functioning of an organ or gland that can make use of the substance.
> What is the half-life of a radioactive nucleus? Does a given type of nucleus always have the same half-life? Do nuclei of different elements have the same half-life?
> At which electrode (anode/cathode) do species gain electrons in a galvanic cell?
> What is an oxidizing agent? Is an oxidizing agent itself oxidized or reduced when it acts on another species?
> The sum of the oxidation states of the atoms in a polyatomic ion is equal to the overall of the ion.
> In assigning oxidation states for a covalently bonded molecule, we assume that the more element controls both electrons of the covalent bond.
> Reduction may be described as a(n) of electrons or as a decrease in .
> Another name for the term oxidation state is .
> Reactions in which one or more are transferred between species are called oxidation–reduction reactions.
> Explain the difference between somatic damage from radiation and genetic damage. Which type causes immediate damage to the exposed individual?
> Although aluminum is one of the most abundant metals on earth, its price until the 1890s made it a “precious metal” like gold and platinum. Why?
> How does an electrolysis cell differ from a galvanic cell?
> What is meant by a nuclear bombardment process? Give an example of such a process, and describe what the net result of the process is.
> The “Chemistry in Focus” segment Stainless Steel: It’s the Pits discusses the fact that stainless steel can corrode if there is a deficit of chromium. How does chromium protect stainless steel?
> Pure iron ordinarily rusts quickly, but steel does not corrode nearly as fast. How does steel resist corrosion?
> In a breeder nuclear reactor, nonfissionable is converted to fissionable .
> is the process of returning metals to their natural state—the ores from which they were originally obtained. This process involves of the metal.
> What are some advantages of using lithium ion batteries in electrical devices? What is a disadvantage?
> Write the chemical equation for the overall cell reaction that occurs in a lead storage automobile battery. What species is oxidized in such a battery? What species is reduced? Why can such a battery be “recharged”?
> Consider the oxidation–reduction reaction Zn(s) + Pb2+(aq) Zn2+(aq) + Pb(s) Sketch a galvanic cell that uses this reaction. Which metal ion is reduced? Which metal is oxidized? What half-reaction takes place at the anode in the
> Consider the oxidation–reduction reaction Al(s) + Ni2+(aq) Al3+(aq) + Ni(s) Sketch a galvanic cell that makes use of this reaction. Which metal ion is reduced? Which metal is oxidized? What half- reaction takes place at the anode in
> What type of reaction takes place at the cathode in a galvanic cell? At the anode?
> In which direction do electrons flow in a galvanic cell, from anode to cathode or vice versa?
> In each of the following reactions, identify which element is being oxidized and which is being reduced by assigning oxidation numbers. a. 4KClO3(s) + C6H12O6(s) / 4KCl(s) + 6H2O(l) + 6CO2(g) b. 2C8H18(l) + 25O2(g) / 16CO2(g) + 18H2O(l) c. PCl3(g) + Cl2
> During nuclear , a large nucleus is transformed into lighter nuclei. During nuclear , small nuclei are combined to make a heavier nucleus. Both processes release energy, but nuclear
> How do the forces that hold an atomic nucleus together compare in strength with the forces between atoms in a molecule?
> Nitric acid is a very strong acid, but is also a very strong oxidizing agent, and generally behaves as the latter. It will dissolve many metals. Balance the following oxidation–reduction reactions of nitric acid. a. Cu(s) + HNO3(aq) Cu2+(
> Each of the following nuclides is known to undergo radioactive decay by production of an alpha particle, /. Write a balanced nuclear equation for each process. a. / b. / c. /
> Each of the following nuclides is known to undergo radioactive decay by production of a beta particle, /. Write a balanced nuclear equation for each process. a. /C b. / c. /
> Complete each of the following nuclear equations by supplying the missing particle. a. 80 201
> How is 6 14
> Naturally occurring magnesium consists primarily of three isotopes, of mass numbers 24, 25, and 26. How many protons does each of these nuclides contain? How many neutrons does each of these nuclides contain? Write nuclear symbols for each of these isoto
> Technetium-99 has been used as a radiographic agent in bone scans ( 43 99
> Although naturally occurring potassium consists mostly of the isotope of mass number 39 (93.25%), isotopes of mass number 41 (6.73%) and 40 (0.01%) also are present. Write the nuclear symbol for each of the isotopes of potassium. How many neutrons are pr
> In each of the following reactions, identify which element is being oxidized and which is being reduced by assigning oxidation states. a. 2Cu(s) + S(s) / Cu2S b. 2Cu2O(s) + O2(g) / 4CuO(s) c. 4B(s) + 3O2(g) / 2B2O3(s) d. 6Na(s) + N2(g) / 2Na3N(s)

