Question: For each of the following atomic numbers,
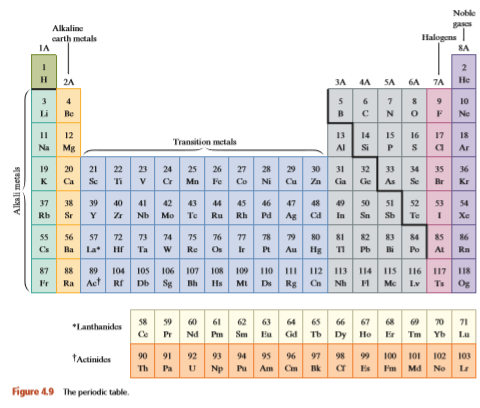
For each of the following atomic numbers, write the name and chemical symbol of the corresponding element. (Refer to Figure 4.9.)
a. 7
b. 10
c. 11
d. 28
e. 22
f. 18
g. 36
h. 54
Transcribed Image Text:
Noble Alkaline carth metals IA Halogens| 8A 2 3A 4A SA 6A 7A H. 2A He 10 Li Be Ne 13 14 15 16 17 II 12 I8 Transition metals Na Mg Si P Ar 25 26 27 v C Mn Fe Co 29 30 31 32 Cu Zn Cia 19 20 21 22 23 24 28 33 34 35 36 K Ca Se Ti Ni Ge As Se Br Kr 37 38 39 40 41 42 43 44 45 46 47 48 50 51 52 53 54 Rb Sr Nb Mo Te Ru Rh Pd Ag Ca In Sn Sb Te Xe 56 57 72 73 74 75 La* Hr 77 78 » s0 81 82 83 Au lg 55 76 84 85 86 Os Ir Pb i Cs Ba Ta Re Po At Rn 112 113 114 Rg Cn Nh 87 88 89 105 106 107 108 109 110 IIS 116 117 Og 104 II 118 Ir Ra Aet Rr Db Sg Bh Hs MI Ds Mc Lv Ts 58 9 60 61 Ce 63 64 65 66 67 8 9 70 Gd Tb Dy Ho 62 71 *Lanthanides Pr Nd Sm Eu Er Tm Yb Lu tactinides 92 93 95 97 99 100 102 90 91 94 96 98 101 103 Th Pa Np Pu Am Cm Es Fm Md No Le Figure 4.9 The periodic table. 星 = 2 Alkali metals
> The processes of melting and evaporation involve changes in the of a substance.
> How many digits after the decimal point should be reported when the calculation (10,434 -9.3344) is performed?
> When the sum 4.9965 + 2.11 + 3.887 is calculated, to how many decimal places should the answer be reported? You should not need to perform the calculation.
> Which of the following are true? a. P4 is considered a compound. b. Metal rusting on a car is a chemical change. c. Dissolving sugar in water is a chemical change. d. Sodium chloride (NaCl) is a homogeneous mixture.
> When the calculation (2.31)(4.9795 * 103)/(1.9971 * 104) is performed, how many significant digits should be reported for the answer? You should not need to perform the calculation.
> The following water measurements are made: 18 mL of water measured with a beaker, 128.7 mL of water measured with a graduated cylinder, and 23.45 mL of water measured with a buret. If all of these water samples are then poured together into one container
> Consider the calculation indicated below: Explain why the answer to this calculation should be reported to only two significant digits. 2.21 x 0.072333 × 0.15 4.995
> Round off each of the following numbers to the indicated number of significant digits, and write the answer in standard scientific notation. a. 0.00034159 to two digits b. 103.351 * 102 to five digits c. 17.9915 to one digit d. 3.365 * 105 to four di
> When a large or small number is written in standard scientific notation, the number is expressed as the product of a number between 1 and 10, multiplied by the appropriate power of 10. For each of the following numbers, indicate what power of 10 would be
> Round off each of the following numbers to the indicated number of significant digits and write the answer in standard scientific notation. a. 4341 * 102 to three significant digits b. 93.441 * 103 to three significant digits c. 0.99155 * 102 to four
> Round off each of the following numbers to two significant digits, and express the result in standard scientific notation. a. 1,566,311 b. 2.7651 * 10-3 c. 0.07759 d. 0.0011672
> Round off each of the following numbers to three significant digits, and express the result in standard scientific notation. a. 254,931 b. 0.00025615 c. 47.85 * 103 d. 0.08214 * 105
> In a multiple-step calculation, is it better to round off the numbers to the correct number of significant figures in each step of the calculation or to round off only the final answer? Explain.
> When we round off a number, if the number to the right of the digit to be rounded is greater than 5, then we should
> Indicate the number of significant figures implied in each of the following statements: a. The population of the United States in 2016 was 324 million. b. One minute is equivalent to 60 seconds. c. There are 1.6093 kilometers in 1 mile. d. The averag
> Indicate the number of significant figures in each of the following: a. 250. b. 250 c. 2.5 * 102 d. 250.0
> Why can the length of the pin shown in Fig. 2.5 not be recorded as 2.850 cm?
> Classify the following mixtures as heterogeneous or homogeneous. a. soil b. mayonnaise c. Italian salad dressing d. the wood from which the desk you are studying on is made e. sand at the beach
> Classify the following as mixtures or pure substances. a. a multivitamin tablet b. the blue liquid in your car’s windshield reservoir c. a ham and cheese omelet d. a diamond
> When a large or small number is written in standard scientific notation, the number is expressed as the product of a number between 1 and 10, multiplied by the appropriate power of 10. For each of the following numbers, indicate what number between 1 and
> What were the four fundamental substances postulated by the Greeks?
> For each of the following chemical symbols, give the name of the corresponding element. a. K b. Ge c. P d. C e. N f. Na g. Ne h. I
> When a measuring scale is used properly to the limit of precision, the last significant digit recorded for the measurement is said to be uncertain. Explain.
> Use the periodic table inside the front cover of this book to find the symbol or name for each of the following elements. Symbol Name zirconium Cs selenium Au cerium
> Use the periodic table shown in Fig. 4.9 to find the symbol or name for each of the following elements Symbol Name Co rubidium Rn radium U
> Observations may be either qualitative or quantitative. Quantitative observations are usually referred to as measurements. List five examples of qualitative observations you might make around your home or school. List five examples of measurements you mi
> In science, what is the difference between a law and a theory? Provide examples of each.
> Being a scientist is very much like being a detective. Detectives such as Sherlock Holmes or Miss Marple perform a very systematic analysis of a crime to solve it, much like a scientist does when addressing a scientific investigation. What are the steps
> Give the symbols and names for the elements whose chemical symbols consist of only one letter.
> Read the “Chemistry in Focus” segment Trace Elements: Small but Crucial, and answer the following questions. a. What is meant by the term trace element? b. Name two essential trace elements in the body and list their function(s).
> What are the five most abundant elements (by mass) in the earth’s crust, oceans, and atmosphere?
> What are the three most abundant elements (by mass) in the human body?
> In addition to his important work on the properties of gases, what other valuable contributions did Robert Boyle make to the development of the study of chemistry?
> Which metric system unit is most appropriate for measuring the length of an insect such as a beetle? a. meters b. millimeters c. megameters d. kilometers
> was the first scientist to recognize the importance of careful measurements.
> Chemistry is an intimidating academic subject for many students. You are not alone if you are afraid of not doing well in this course! Why do you suppose the study of chemistry is so intimidating for many students? What about having to take a chemistry c
> Which of the following is(are) correct? a. 40Ca2+ contains 20 protons and 18 electrons. b. Rutherford created the cathode-ray tube and was the founder of the charge-to-mass ratio of an electron. c. An electron is heavier than a proton. d. The nucleus
> Complete the following table. Atom/lon Protons Neutrons Electrons 120s Mg* Fe+ 34De SCu Noble gases Alkaline Halogens is 1 carth metals 1A 8A 2 2A 13 ЗА 14 15 4A SA 16 6A 17 7A H He 3 4 6 7 9. 10 Li Be B Ne 9 10 11 12 3 11 12 13 14 15 16 17 18 Trans
> Using the periodic table, complete the following table. Atoms Number of Protons Number of Neutrons Mn Ni 28 201 Noble gases Alkaline Halogens is 1 carth metals 1A 8A 2 2A 13 ЗА 14 15 4A SA 16 6A 17 7A H He 3 4 6 7 9. 10 Li Be B Ne 9 10 11 12 3 11 12
> Complete the following table to predict whether the given atom will gain or lose electrons in forming an ion. Gain (G) or Lose (L) Electrons Atom lon Formed Mg Rb Br CI ||
> Complete the following table. Number of Protons Number of Neutrons Symbol 34 45 19 20 53 74 5 24 32 4.
> Provide the symbols for the elements given in the following table. Element Name Symbol tin beryllium hydrogen chlorine radium хenon zinc охудen
> Provide the name of the element that corresponds to each symbol given in the following table. Symbol Element Name Au Kr Не Li Si
> Read the “Chemistry in Focus” segment A Four-Wheel-Drive Nanocar. It discusses carbon and copper atoms. Both atoms have stable isotopes. Example 4.2 had you consider the isotopes of carbon. Copper exists as copper-63 and copper-65. Determine the number o
> The unit of volume in the metric system is the liter, which consists of 1000 milliliters. How many liters or milliliters is each of the following common English system measurements approximately equivalent to? a. a gallon of gasoline b. a pint of milk
> For each of the following elements, use the table shown in Fig. 4.9 to give the chemical symbol and atomic number and to specify whether the element is a metal or a nonmetal. Also give the named family to which the element belongs (if any). a. carbon b
> Complete the following table. Symbol Protons Neutrons Mass Number HCa 25 30 47 109 45
> How many protons and neutrons are contained in the nucleus of each of the following atoms? In an atom of each element, how many electrons are present? a. Ti b. Zn Ge d. Kr 86 64 30 e. As 75 е. 76 с. 32 f. ÝK 41 19
> Write the atomic symbol /for each of the isotopes described below. a. Z = 6, number of neutrons = 7 b. the isotope of carbon with a mass number of 13 c. Z = 6, A = 13 d. Z = 19, A = 44 e. the isotope of calcium with a mass number of 41 f. the isoto
> Write the simplest formula for each of the following substances, listing the elements in the order given. a. a molecule containing one carbon atom and two oxygen atoms b. a compound containing one aluminum atom for every three chlorine atoms c. perchl
> For each of the following chemical symbols, give the name of the corresponding element. a. Te b. Pd c. Zn d. Si e. Cs f. Bi g. F h. Ti
> Give the chemical symbol for each of the following elements. a. silver b. aluminum c. cadmium d. antimony e. tin f. arsenic
> Which of the following series of elements is not matched with the correct description? a. F, Cl, Br—halogens b. He, Ne, Ar—noble gases c. Mg, Ca, Sr—alkaline earth metals d. Fe, Co, Ni—transition metals e. B, Si, Ge—metals
> Give the chemical symbol for each of the following elements. a. barium b. potassium c. cesium d. lead e. platinum f. gold
> Which English unit of length or distance is most comparable in scale to each of the following metric system units for making measurements? a. a centimeter b. a meter c. a kilometer
> How many electrons are present in each of the following ions? a. Se2- b. Br- c. Cr3+ d. Rb+ e. Bi3+ f. Cu2+
> How did Robert Boyle define an element?
> Though the common isotope of aluminum has a mass number of 27, isotopes of aluminum have been isolated (or prepared in nuclear reactors) with mass numbers of 24, 25, 26, 28, 29, and 30. How many neutrons are present in each of these isotopes? Why are the
> How many protons and neutrons are contained in the nucleus of each of the following atoms? For an atom of the element, how many electrons are present? 63 a. 29 Cu SCu b. Br 24 с. Mg 35
> When iron rusts in moist air, the product is typically a mixture of two iron–oxygen compounds. In one compound, there is an equal number of iron and oxygen atoms. In the other compound, there are three oxygen atoms for every two iron atoms. Write the for
> Carbohydrates, a class of compounds containing the elements carbon, hydrogen, and oxygen, were originally thought to contain one water molecule (H2O) for each carbon atom present. The carbohydrate glucose contains six carbon atoms. Write a general formul
> Which of the following is(are) true regarding 37Cl- and 40Ar? a. same group number on the periodic table b. same number of protons c. same number of neutrons d. same number of electrons
> Which of the following statements is(are) true? a. Dalton was the first to theorize that atoms consist of smaller particles called electrons, protons, and neutrons. b. Dalton’s atomic theory didn’t account for isotopes. c. All particles in the nucleus
> Give the group number (if any) in the periodic table for the elements listed in Problem 85. If the group has a family name, give that name. From problem 85: For each of the following elements, give the chemical symbol and atomic number. a. astatine b.
> For each of the following elements, give the chemical symbol and atomic number. a. astatine b. xenon c. radium d. strontium e. lead f. selenium g. argon h. cesium
> The fundamental SI unit of length is the meter. However, we often deal with larger or smaller lengths or distances for which multiples or fractions of the fundamental unit are more useful. For each of the following situations, suggest what fraction or mu
> For each of the following negative ions, use the concept that a chemical compound must have a net charge of zero to predict the formula of the simple compounds that the negative ions would form with the Cs+, Ba2-, and Al3- ions. a. I- b. O2- c. P3- d
> For each of the following positive ions, use the concept that a chemical compound must have a net charge of zero to predict the formula of the simple compounds that the positive ions would form with the Cl2, S-2, and N3- ions. a. K+ c. Al3+ e. Li+ b. M
> Why must the total number of positive charges in an ionic compound equal the total number of negative charges?
> Why does an ionic compound conduct an electric current when the compound is melted but not when it is in the solid state?
> Why does a solution of sodium chloride in water conduct an electric current?
> List some properties of a substance that would lead you to believe it consists of ions. How do these properties differ from those of nonionic compounds? Source of electric power Salt dissolved in water
> On the basis of the element’s location in the periodic table, indicate what simple ion each of the following elements is most likely to form. a. P b. Ra c. At d. Rn e. Cs f. Se
> For each of the following atomic numbers, use the periodic table to write the formula (including the charge) for the simple ion that the element is most likely to form. a. 53 b. 38 c. 55 d. 88 e. 9 f. 13
> For the following ions, indicate whether electrons must be gained or lost from the parent neutral atom, and how many electrons must be gained or lost. a. O2+ b. P3- c. Cr3+ d. Sn2+ e. Rb+ f. Pb2+
> For the following processes that show the formation of ions, use the periodic table to indicate the number of electrons and protons present in both the ion and the neutral atom from which the ion is made. а. Са — Са?+ + 2e- b. Р+ Зе-— р3- Br- с. Br
> Who is taller, a man who is 1.62 m tall or a woman who is 5 ft 6 in. tall?
> State the number of protons, electrons, and neutrons for /
> How many electrons are present in each of the following ions? a. Ba+2 b. P3- c. Mn2+ d. Mg2+ e. Cs+ f. Pb2-
> True or false? N-3 and P-3 contain a different number of protons but the same number of electrons. Justify your answer.
> Based on their location in the periodic table, give the symbols for three elements that would be expected to form positive ions in their reactions.
> Simple negative ions formed from single atoms are given names that end in .
> Positive ions are called , whereas negative ions are called .
> An ion that has two more electrons outside the nucleus than there are protons in the nucleus will have a charge of .
> A simple ion with a 3+ charge (for example, Al+3) results when an atom (gains/loses) electrons.
> Ions are produced when an atom gains or loses .
> An isolated atom has a net charge of .
> The tablecloth on my dining room table is 2 m long, which is cm or about in.
> The two most common elemental forms of carbon are diamond and .
> Most of the elements are solids at room temperature. Give three examples of elements that are liquids at room temperature, and three examples of elements that are gases at room temperature.
> If sodium chloride (table salt) is melted and then subjected to an electric current, elemental gas is produced, along with sodium metal.
> A simple way to generate elemental hydrogen gas is to pass through water.
> Give three examples of gaseous elements that exist as diatomic molecules. Give three examples of gaseous elements that exist as monatomic species.
> Molecules of nitrogen gas and oxygen gas are said to be , which means they consist of pairs of atoms.
> Why are the elements of Group 8 referred to as the noble or inert gas elements?
> The noble gas present in relatively large concentrations in the atmosphere is .
> Are most of the chemical elements found in nature in the elemental form or combined in compounds? Why?
> Most substances are composed of rather than elemental substances.
> The GPS in my car indicates that I have 100. mi left until I reach my destination. What is this distance in kilometers?
> The “Chemistry in Focus” segment Putting the Brakes on Arsenic discusses the dangers of arsenic and a possible help against arsenic pollution. Is arsenic a metal, a nonmetal, or a metalloid? What other elements are in the same group on the periodic table

